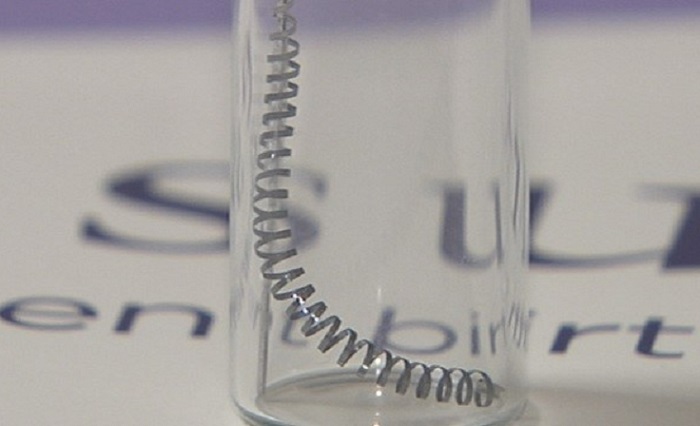
The Essure medical device consists of metal coils that are inserted into a woman’s Fallopian tubes in an attempt to prevent conception. This product was intended to be a non-surgical method of sterilization, and it is the only one of its kind to be approved by the Food and Drug Administration (FDA). As of the end of 2018, thousands of adverse events had been reported by patients, physicians and other healthcare providers because of Essure. The side effects reported were often severe, some involving multiple surgical procedures to remove the metal coils. As a result, the product was under scrutiny for its safety and effectiveness for a long time. Despite the scrutiny, the product manufacturer Bayer expressed that they stood by the safety and efficacy of the device.
Essure Side Effects
In 2015, it was estimated that thousands of Essure patients required operations to correct the device’s faults in the past several years. Apparently, the device would travel from the implantation location and tear through the uterus or other nearby organs. Additionally, the scar tissue that was supposed to form around the device after implantation and prevent pregnancy, sometimes became extensive, and as a result, some women developed autoimmune disorders that was attributed to the immune reaction induced by the device.
Thousands of women sought legal advice to take action against the manufacturer over not only the major adverse events, but the sterilization failure rates as well. Nearly 10% of users are at risk of unplanned pregnancy when Essure is their sole method of birth control, according to researchers. Tubal ligation, a more traditional method of sterilization, has a failure rate of 1 out of every 1,000 women in the first year alone. Some of these women who experience unintended pregnancies while using Essure also endured miscarriages, stillbirths, and ectopic pregnancies.
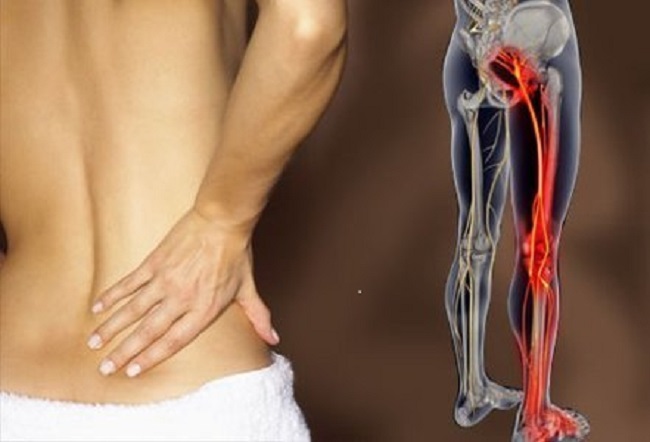
Aside from the tears and perforations, Essure has several other reported side-effects that women often experience. Immediately following the procedure, women commonly reported mild to moderate pain or discomfort. Within a few days, women also report headaches, weight fluctuation, fatigue, depression, pain with sex, abdominal pain, back pain, severe pelvic pain, as well as severe menstrual cramps. Some women say these go away within a few weeks or months, but a lot of women experience this pain chronically.
In the summer of 2018, the product’s manufacturer, Bayer AG, announced they were voluntarily discontinuing sales of the device. This was after the FDA restricted sales and distribution of the device, as well as stepped in many times to regulate the product.
How Essure Was Approved
The FDA requires a Premarket Approval (PMA) to review and evaluate the safety and effectiveness of Class III medical devices like Essure. FDA regulations require that this takes 180 days, and normally longer, but there are records that show that this process was expedited and several trial candidate’s records were altered to obscure unfavorable data. Some patients were completely thrown out of the clinical trials when their data reflected poorly on the device. According to the citizen’s petition linked above, there were many adverse events that Essure manufacturers and PMA holders failed to report and/or covered up. Additionally, over 16,000 patient complaints were withheld from the FDA. When the manufacturing facilities were inspected, many unsettling thing came to light, starting with the facility’s lack of license. The manufacturer of Essure were operating without a license for three years.
In recent years, the FDA began enforcing label changes, as well as other methods to regulate the product and its potential risks. In 2016, they required a boxed warning to be placed on the product to warn providers of the risks that could come with Essure implantation. Black box warnings are the most serious warnings required by the FDA and indicate the severity of the risks of the medication. Another major step in regulation was the introduction of a patient decision checklist that would be reviewed before implantation by both the physician and the patient together. When the FDA began regulating sales, they made it so only patients that reviewed this checklist could use Essure.
Device and Regulatory Failure
Thousands of Essure patients have experienced adverse events as a result of the device. Essure was intended to be an easier method of sterilization, as it was non-surgical and required no general anesthesia. The recovery time was supposed to be minimal, and women could return to daily activities the same day of implantation. Instead, thousands of women ended up with chronic pain, and sometimes even children they did not intend to have.
The medical device industry remains largely unregulated to this day. A recent documentary, The Bleeding Edge, highlights the resulting adverse health outcomes that result from this lack of regulation. Several women with Essure failures are interviewed. It is worth watching if you or a loved one is contemplating a medical device.
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, like it, please help support it. Contribute now.













I wonder how many women have had hysterectomies from the damage caused by Essure. I doubt anyone is keeping track. Hysterectomy is another very harmful surgery of which women are not informed by their gynecologists. It is time that the medical professionals and corporations be held accountable for their destructive medical devices and procedures.