Some 20 years ago, during my very first neuro class taught by an accomplished neurologist from a prominent research university, I had a conversation about hormones and the brain. It was a brief conversation during which he admitted not only knowing nothing about how hormones affected the brain or nervous system functioning, but also, how he and others had no interest in considering the question. He believed hormones were too complicated to consider relevant. One didn’t ‘mess with hormones’ as he put it.
Lucky for us, some intrepid neurologists have moved the science of neuroendocrinology past the foibles of ‘don’t mess with hormones’ to hormones might be important therapeutic options. Nowhere is this more evident than in the areas of traumatic brain injury and diseases of demyelination. Here we see advances in hormones used as viable and important treatments where once there were none. Although the research is yet in its infancy and suffers from the typical one-size-fits-all approach, it marks a huge step forward in clinical neuroendocrinology.
What is Neuropathic Pain?
Neuropathic pain, the often chronic and difficult to treat pain that comes from nerve injury and demyelination affects approximately 3% of the population. The number of individuals suffering from neuropathy is likely much higher when one considers diseases such as endometriosis and the ill-understood, under-recognized neuropathy emerging post medication or vaccine adverse reactions. The experience of neuropathic pain in hands, feet, arms and legs is described as burning, freezing, electrical, tingling, prickling and more often than not, severe and unrelenting. As the nerve injury progresses and the pain continues, the rawness and intensity of the pain becomes indescribable to someone who has not experienced nerve pain firsthand.
Hormones and the Nervous System
Since the late eighties, researchers have known that steroid hormones, such as progesterone were not limited to reproductive functions and that many steroids were active in the nervous system. Not only were those steroids synthesized peripherally in ovaries, adrenals or adipose tissue able to cross the blood brain barrier, but all the core substrates for steroid synthesis were available in the brain too, meaning the brain could make its own steroids, de novo, from scratch. Researchers initially deemed steroids made or active in the brain as neurosteroids. Eventually, that nomenclature fell by the wayside as researchers realized there was tremendous crosstalk between peripheral and central hormones, no matter where the hormones were synthesized.
It should be noted, that hormones exert influence all over the body and brain via receptor binding. (A discussion on hormones and receptors can be found here: Promiscuous Hormones and Other Fun Facts.) In addition to steroid hormone receptors on (cell membrane) and in (nuclear) hormone-specific cells, like those in ovary, testes, adrenals, uterus, endometrium, hormone receptors are co-located on neurons, glial cells, oligodendrocytes and Schwann cells (myelin producing cells), immune cells, cardiomyocytes (heart), hepatocytes (liver), adipocytes – essentially every cell, organ or tissue in our body is modulated in some way by a hormone. Hormone influence is particularly important in the in the nervous system, where everything from neurotransmitter release and uptake to synaptic connections are modulated.
Traumatic Brain Injury, Peripheral Neuropathy and Hormones
When we talk about injuries to the nervous system, be it the brain and spinal cord, which is called the central nervous system (CNS), or all of the nerves that control movement and organ function in the body, the peripheral nervous system (PNS), there are two categories of injuries, those that develop acutely, post trauma, or those that develop chronically because of some metabolic dysfunction. In the case of the former, traumatic brain injuries (TBI) or traumatic nerve injuries, the research points to progesterone for repair and regrowth. In the case of the later, where injuries develop as a result of internal and often chronic dysfunction, such as diabetic neuropathy, multiple sclerosis and other diseases affecting nerve fibers and myelin, less is known about progesterone and the thyroid hormone triiodothyronine (T3) is implicated, more strongly.
What is Myelin and How Does it Impact Neuropathy?
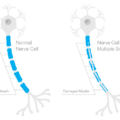
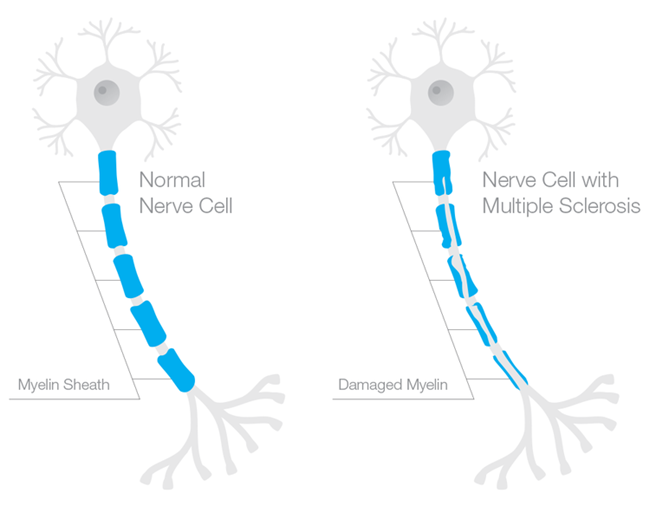
Myelin is the insulation that protects the axons of the neuron (in the brain) or nerve (in the body) to allow rapid conduction or messaging across the brain or through the body. Recall the axon is the part of the neuron/nerve that sends messages to other neurons/nerves, to other tissues, like muscle, or to organs like the heart and the liver. The dendrites receive messages and the nucleus processes messages. Myelin is like the plastic coating around the electrical wiring in your house. If the coating is too thick, conduction is blocked. If the coating is frayed or too thin, electrical sparks fly everywhere. Frayed myelin around axons is one of the mechanisms of neuropathic pain. Myelinated axons in the brain look white and therefore are called white matter. Whereas the grey matter, is where the nuclei of the brain reside. White matter in the brain consists of the oligodendrocytes – the type of cell that forms the myelin sheathing around axons. Myelin in the body, around the peripheral nerves, is made from cells called Schwann cells.
Progesterone, Myelination and the Nervous System
In the 1990s, Etienne-Emil Baulieu and colleagues recognized a role for progesterone (and other hormones) in central nervous system myelination. Over the next two decades, researchers uncovered the possible mechanisms and delineated more clearly for whom and in what types of injury progesterone seems most helpful. From studies of neurons (CNS) nerve cells (PNS), we now know that progesterone is key for myelination and neuron/nerve regrowth, at least in the acute stages. Progesterone stimulates myelination both directly by acting on oligodendrocytes and indirectly via actions on the neurons and the astrocytes that then message the oligodendrocytes to produce more myelin. Similarly in the PNS, progesterone aids in the remyelination and re-growth of nerve fibers, via the Schwann cells and via progesterone receptors located in what are called the dorsal root ganglia (DRG), the sensory neurons that carry information from the periphery to the brain. Whether in the CNS or the PNS, timing and length of progesterone administration are critical.
Animal Research – Progesterone, Nerve Injury and Neuropathy
The animal research has been mixed, but taken together, the results seem dependent upon the type of injury, the timing of the treatment and the methods of assessment. When treatment is begun early enough and extended long enough (this varies) and when the measure is neuropathic pain versus other potential outcomes (such as morphological changes to the nerve), there seems to be a favorable response. In rodents, single dose treatment does not seem to work, neither does treatment that is initiated too late after the injury or ended prematurely, though these criteria vary from study to study.
For example, using an induced model of diabetic neuropathy, researchers from Italy found that diabetes markedly reduced progesterone concentrations in male rodents within three months (females were not tested). This was the only study I could find that measured progesterone concentrations relative to treatment and outcomes. Chronic treatment (one month) with progesterone or one of its derivatives restored nerve function, increased key components of myelin production and reduced pain. Similarly, an induced model of trigeminal pain in male rodents found when progesterone was initiated early and at a high enough dosage, it tempered the experience of pain while increasing myelin producing proteins. Lower dosages did not work.
From Animals to Humans: Traumatic Brain Injury and Neuropathy
The research with animals, male rodents specifically, shows that progesterone treatment works best if given early enough, for long enough, and at high enough dosages. With acute or induced injuries under experimental conditions, early treatment is much easier than in real life where neuropathic pain develops much more gradually and often goes undiagnosed and untreated for some time. Would progesterone work in humans and would it work for chronic, well established neuropathy? The answers to those questions are not clear because the human research on progesterone and myelin focuses on acute injury, like the traumatic brain injuries. The human research also suffers from short duration dosing, includes mostly males, and without exception fails to address endogenous progesterone concentrations either pre or post treatment. Nevertheless, there are some indications that progesterone therapy may work.
Progesterone and TBI – Human Studies
In a smaller, single center open trial and two larger, double-blind, placebo-controlled, human trials, progesterone therapy was administered to individuals with severe traumatic brain injuries (Glasgow coma scale <8). In each case, the progesterone group did better, showed reduced morbidity rates than the placebo groups.
In the first study, 26 cases were treated with progesterone and 20 controls with placebo. At both 10 days and three months post injury and treatment, the progesterone treated group improved significantly more than the control group (abstract only).
In a second study, 159 patients, arriving to the treatment facility just eight hours post traumatic brain injury were randomized to receive either intramuscular injections of progesterone (82) or placebo at 1.0 mg/kg via intramuscular injection and then once per 12 hours for 5 consecutive days. Both intake neurological functioning and post treatment functioning were assessed and compared using a number of measures. Follow up assessment was conducted at 3 and 6 months post injury/treatment. The results were positive, albeit small. The progesterone treated group improved significantly across all measures showing consistently larger improvements compared to the placebo group. It should be noted that only 44 of the total subject population was female, 24 in the placebo group and 20 in the progesterone group. No analysis by sex was conducted and so it is not clear whether progesterone therapy works equally well in males and females.
In the third study, called ProTECT, a similar double-blind, placebo controlled, randomized methodology was used. Here, however, the randomization was 4:1 and favored progesterone treatment, whereas in the study cited above, the progesterone and placebo randomization was 1:1. Progesterone was given via IV for three days. The ProTECT study researchers found that patients in the progesterone had a lower 30-day mortality rate than controls (rate ratio 0.43; 95% confidence interval 0.18 to 0.99). While those who suffered more severe injuries had relatively poor outcomes at the follow up tests 30 days post injury, despite the treatment, and those who suffered only moderate traumatic brain injury and received progesterone were more likely to have a moderate to good outcome than those randomized to placebo (abstract only).
Two additional trials are on-going, hoping to test progesterone on thousands of patients: the ProTECT-III and SynAPSe studies.
Translating the TBI Research for Use with Neuropathy
What does improvement post TBI tell us about treating neuropathic pain from demyelination disorders? It is not clear, because even though researchers know that progesterone promotes myelination, the human research has focused narrowly on injuries where demyelination occurs but also where other factors are also involved in the outcome. We know from animal and cell culture research that progesterone attenuates the cascade of events that occur post TBI or post nerve injury via multiple mechanisms, inducing myelin regrowth is only one of those mechanisms. Progesterone reduces swelling of both vasogenic and cytotoxic sorts. It has anti-oxidant properties, upregulating enzymes that increase free radical elimination. Progesterone inhibits inflammation, stabilizes mitochondria, reduces neural excitoxicity and can limit apoptosis. Finally, progesterone promotes myelination. All factors that should point to consistent improvement in TBI and neuropathic pain syndromes, but the research is limited and mixed. Why?
The primary reason for mixed results is study design, almost all are short duration. Hormones are long acting molecules and the shorter duration may not be sufficient to generate the response, particularly when the injuries are severe or longstanding. Longer treatment regimes are likely in order.
Another reason for mixed results is the one-size-fits-all approach. None of the human studies and few of the animal studies, investigates why progesterone works in some subjects and not others. Almost all of the studies are predominantly male, rodent and human alike. None have investigated whether being female has anything to do with efficacy. None of the human studies measured circulating concentrations of progesterone, either pre-, during, or post-treatment and so there is no way to tell if those who responded had higher circulating concentrations or if improvement was contingent upon reaching a certain concentration.
Perhaps even more importantly, is the fact that progesterone, like any hormone, works within a vast and compensatory network of other hormones. The reductionist approach that utilizes a single hormone treatment protocol, while ignoring the potential cross-talk with other hormones and other variables is a consistent flaw these and other research protocols. Again, hormone measurement, progesterone and its metabolites, in addition to other key hormones, is imperative if one is to determine therapeutic efficacy.
I Have Peripheral Neuropathy, Should I Try Progesterone?
Progesterone therapy is generally safe, but as with everything there are risks. Women have been using it for generations in its bio-identical form to mitigate menstrual and menopausal symptoms. Since it is fat soluble, transdermal (skin) absorption is possible and progesterone creams have become popular. Some physicians prefer micronized progesterone, a pill form that reduces the molecule so it more easily passes through the liver without degradation. The pill form, and to a much lesser degree, transdermal progesterone, cause sedation and should be taken at night. Micronized progesterone has been shown to increase free thyroxine (T4) as well. For some women, and presumably men too, a gain of function mutation on the mineralocorticoid receptor can evoke very high blood pressure with any increase in progesterone concentrations (luteal phase of the menstrual cycle and during pregnancy especially). Although there are dosing references for progesterone relative to menstrual or menopausal therapy, the dosing is individualized and often includes the replacement of other hormones along with progesterone. Salivary hormone testing is used to monitor and hormone doses are adjusted regularly. Progesterone is also used predominantly for women. No such dosing considerations exist for men that I am aware of. Likewise, for peripheral neuropathy there are no references from which to design a treatment protocol and so it would be prudent to work with a functional medicine specialist, familiar with hormone management, to develop and monitor the course of treatment.
My Two Cents
I suspect, if progesterone therapy works for peripheral neuropathy, it will require a much longer term treatment period than is currently tested in the human trials. I suspect also, it will be difficult to ascertain whether it is the sole contributor to improvements in neuropathy symptoms, as neuropathy is a multi-factorial process that ought to be treated as such. Nevertheless, if you suffer from neuropathy and can find a physician to work with that is familiar with hormones and the research, progesterone therapy might provide a viable option, among other options like stabilizing thyroid hormones and supporting mitochondrial function.
Postscript
This article was first published February 19, 2014. Since then, a few more studies and review articles have been published and continue to support the role of progesterone in myelin regeneration, although the data are mixed. From a 2020 article.
Indeed, PROG and its metabolites modulate the expression of myelin proteins of the PNS, such as myelin basic protein (MBP), myelin proteolipid protein, glycoprotein zero (P0) and peripheral myelin protein (PMP22) as well as myelin formation [6,8,9,20,63,64]. In particular, the expression of P0 in the sciatic nerve of adult male rats, as well as that in Schwann cell culture, is increased by treatment with PROG, DHP or THP.
…Data here reported support the concept that neuroactive steroids, synthetic ligands acting on their receptors or inducing their synthesis, may improve PN symptoms, including neuropathic pain and consequently may represent an interesting possible therapeutic strategy. In addition, based on the sexual dimorphism of neuroactive steroids as well as of PN here discussed, a gender specific treatment based on these compounds may be also proposed.
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
This article was published originally on February 19, 2014.