Reproductive senescence, the time in a woman’s life marked by the slowing and eventual cessation of reproductive function, frequently coincides with an increased risk of a host of neurodegenerative disorders from memory impairment to dementia and Alzheimer’s disease. Researchers have long postulated that the loss of ovarian hormones was responsible; estradiol, in particular, but likely others as well.
This begs the question, what happens to the brain when we abruptly and artificially derail ovarian hormone synthesis in young women using drugs such as Lupron (leuprolide) and the other GnRH agonists and antagonists or by removing the ovaries altogether as in surgical oophorectomy? Is it the same damage we see in aging, only expedited and perhaps magnified, or does it run a different course? Along those same lines, though perhaps a topic for another day, what happens when we chronically supplant endogenous ovarian hormone production with synthetic hormones such as those used in hormonal birth control or menopausal hormone replacement therapies? I suspect, and there is evidence to back up my suspicions, that in all cases brain function is altered, and not for the better.
Estrogens and the Brain
The mechanisms by which estradiol and other steroid hormones influence brain function are myriad and complicated. Beyond just reproduction, steroid hormones influence all aspects of neurological function, with estrogen, androgen, glucocorticoid (cortisol), and mineralocorticoid (electrolyte balance, blood pressure) receptors located throughout the brain. Steroid hormones produced in the body, because of their fat solubility, easily cross the blood-brain barrier where they bind to their receptors and regulate all sorts of processes. Perhaps even more remarkable, the brain has all of the machinery to synthesize its own steroid hormones and so when body concentrations fall, at least for a time, the brain can compensate. Eventually, however, brain synthesis declines and that is where we begin to have problems. Fortunately, natural reproductive senescence occurs later in life and the risk of neurodegenerative diseases is just that, a risk, not a foretold conclusion. This suggests that other variables are at play, ones that we may be able to modulate to improve health, offset and/or reduce the severity of the natural neurodegenerative processes. Again, however, we must ask, what happens when we induce reproductive senescence in young women? By all accounts, the effects are often devastating, leaving many to wonder if they will ever recover.
Estradiol and Mitochondrial Energy
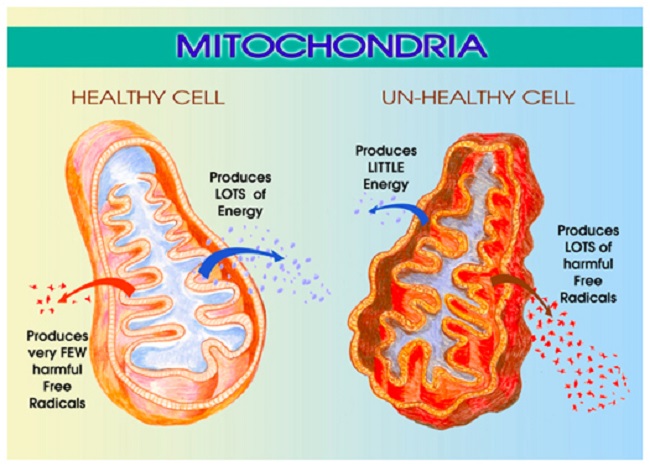
Among the myriad of functions mitochondria control, perhaps the most important is energy production. That is, mitochondria take the nutrients supplied by diet and convert them into adenosine triphosphate (ATP), the energy currency that cells use to perform all of the functions that keep us alive. The loss or diminishment of ATP is deleterious to health and can ultimately be deadly, by invoking a series of complicated processes
Estradiol is a critical component of that process and directly impacts mitochondrial energy production. That’s right, estradiol is part of the mitochondrial bioenergetic machinery such that when estradiol wanes, so too does energy production or ATP. As one might suspect, waning ATP is deleterious to brain health. In previous posts, I detailed the research showing how the loss of estradiol deforms mitochondrial morphology essentially disabling mitochondrial membrane potential while turning the mitochondria into misshapen donuts and blobs ripe for a slow, messy necrotic death; a process that evokes all sorts of deleterious reactions.
The Lupron Brain and Ketosis
Just recently, I stumbled upon research showing yet another mechanism of damage. In the absence of estradiol, brain glucose transport diminishes significantly. This effectively starves the brain for energy inducing severe bioenergetic deficiencies with all of the concordant neuronal damage one might expect. The reduction in glucose affects the mitochondria severely. Recall that glucose is one of the major fuel substrates of the brain, particularly where the Western diet predominates. The decline of glucose transport, therefore, is significant, and alone, without any other changes to the mitochondria, elicits a cascade of deleterious reactions. Oxidative phosphorylation and associated enzymes are downregulated, ATP production wanes, and ultimately may initiate the deformation of the very shape of the mitochondria, as observed in the research cited above. The ensuing reduction of ATP starves the brain of critical energy but also induces a state of hypoxia with the mitochondria incapable of utilizing molecular oxygen. With that hypoxia, inflammatory pathways are initiated further cementing mitochondrial death spirals and associated neuronal damage.
Interestingly, this reduction in aerobic activity coincides with the emergence of a ketogenic phenotype. That is, with the loss of one fuel substrate, ketones become the dominant source of fuel and the associated enzyme machinery is upregulated. Unfortunately, the Western diet is highly dependent upon carbohydrates and so a woman experiencing this loss of estradiol is not likely to consume sufficient fats and proteins to effectively weather this shift. Nevertheless, it does provide an opportunity for recovery. What if women who have lost the ability to produce sufficient estradiol either because of surgically (oophorectomy) or chemically (Lupron and other GnRH analogs) induced menopause adopt a ketogenic diet? Could we maximize the preferred energy source of the post-menopausal brain and reduce the neurological symptoms? I do not know the answer to that question, but given the severity of the suffering with surgical and chemical menopause, it seems worth the try.
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, and like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
Image by Angelo Esslinger from Pixabay.
This article was originally published on November 15, 2018.