Recent attention in a dual Reveal and Kaiser Health News Report (‘Kaiser Report’) to the risks of Lupron’s use in children with central precocious puberty or growth issues, and to Lupron’s risks in general, presents an opportunity to continue the disclosures on the risks of Lupron. This is the third part in a 6-week series exploring numerous areas addressing the use of Lupron in the pediatric and teen population (part 1, part 2).
Lupron and Reproductive Injury
The original patent for Lupron (leuprolide), granted in 1977, was for ovulation induction. Because of Lupron’s hazardous drug status and its categorization by the FDA as a Pregnancy Category X drug (any woman who is or who may become pregnant should avoid because of risk to fetus), it could not gain FDA approval for the indication of ovulation induction. Therefore, Lupron’s manufacturer sought, and gained, FDA approval for use in palliative treatment of prostate cancer. This then allowed the drug to be prescribed off-label for ovulation induction and many other unapproved indications. Over the next several decades, Lupron’s use has expanded into multiple areas of pediatric and women’s health. There are three FDA approved indications (‘precocious puberty’ in children, ‘pain management in endometriosis’ and ‘the hematologic management of anemia associated with fibroids when iron therapy alone is ineffective’), and many off-label uses of Lupron (see Incomplete A-Z List of Off-Label Uses here).
In 1983, ten years before Lupron received FDA approval for precocious puberty (PP), GnRHas were being tested extensively in a variety of indications, including “as a new treatment for idiopathic precocious puberty”, and for male and female contraception. Eleven years later a pilot study using Lupron plus low-dose estrogen as a preventative for breast cancer was deemed “an adventure into the unknown”, and the FDA determined that this treatment “should not move into larger clinical trials” (The Pink Sheet 1994; 56(27):13. ‘GnRH/low-dose Steroids Not Appropriate for Study in Breast Cancer High Risks’). The FDA Committee Chairman said at the time:
“It would be better to recommend a study of the drugs in a high-risk population as a chemopreventative for a long time, find out what its long-term effects were, and then consider it for a larger population.”
In 1993, the year of Lupron’s FDA approval for PP, a study was published of 10 girls who had been followed up to 5 years, and while concluding Lupron was safe and effective, it noted:
“[l]onger-term studies, including reproductive history, will be needed before the potential effects of treatment on fertility can be assessed.”
The following year, in 1994, the FDA recommended nonclinical safety studies of GnRH analogs be conducted. And while it is not clear whether these nonclinical safety studies were conducted, one FDA Medical Office stated at the time:
“the possibility exists that some germ cells may have been permanently affected by drug treatment. It is therefore important to investigate the effects on fetal morphology (teratogenicity) and on postnatal development of the offspring.”
In a long-term clinical study in 1999 examining GnRHa treated girls with PP (Lupron being the most frequently prescribed GnRHa), it was identified that:
“Ovarian volumes tend to increase progressively over the first 3 posttreatment years and were often larger than normal by 3 year post-therapy [and these findings] suggest that recovery of the suppressed gonad of girls treated for longer periods of time may be a more gradual process, and that a complete picture of the effects of therapy may only emerge after several years have passed.”
Similarly, the original rat studies provided to the FDA for its initial 1985 approval in prostate cancer identified that
“[t]he severity of the lesions were greater in testes of rats sacrificed 7 days after cessation of [Lupron] indicating that the effects continued after drug withdrawal (emphasis mine)”. (“Review and Evaluation of Pharmacology and Toxicology Data‟, NDA [New Drug Application] 19-010, March 1, 1984.)
Lupron Depot-PED’s label states “[f]ollowing subcutaneous administration of LUPRON DEPOT to male and female rats before mating there was atrophy of the reproductive organs and suppression of reproductive performance.” The label also states “[f]ollowing a study with leuprolide acetate, immature male rats demonstrated tubular degeneration in the testes even after a recovery period.” (Even though these rats failed to recover histologically, the label claims they were as fertile as the controls.”)
Precocious Puberty, Lupron, and PCOS
In a 2010, Italian study of girls with early puberty treated with GnRHas, the prevalence of polycystic ovary syndrome (PCOS) and hyperandrogenemia “was significantly higher” than those untreated, and “this study represented the first evidence of an independent effect of GNRHa treatment in increasing the risk for PCOS during adolescence in girls with early puberty.” At least one earlier study noted “very large ovaries” when GnRHa treatment was stopped, and subjects “had an increased prevalence of ovaries with a polycystic appearance.” PCOS has been associated with increased risk of metabolic syndrome, diabetes, and dyslipidemia – conditions which may increase risk of cardiovascular disease. PCOS is also associated with infertility, which can result in the need for assisted reproductive technologies which often involve the use of Lupron.
Lupron and Torsion
In a Brazilian case report of a girl with McCune Albright syndrome (which, though rare, accounts for about 10% of PP cases), a salpingo-oophorectomy (surgical removal of fallopian tube and ovary) was required after the 3rd leuprolide dose due to complete torsion of right adnexa and a necrotic, cystic right ovary. This case report also notes that:
“treatment [for McCune Allbright syndrome] with a GnRH analog can result[] in ovarian stimulation, cyst formation, increase of [ovarian] volume and adnexal torsion” requiring surgical removal of gonads. (See photo of this girl’s enlarged, cystic, necrotic ovary in case report’s “Figure 2”.)
In a review of FDA’s adverse event reports (“AERS”), data valid through June 2016, for “Lupron Depot-PED”, “Lupron (leuprolide; daily injection), and “Lupron Depot”, there were no reports found for “salpingo-oophorectomy”. The above published case of a pediatric salpingo-oophorectomy should have been reported, both to the drug company and subsequently to the FDA. The case of ovarian torsion and of ovarian necrosis that appears in a ‘Lupron Depot-PED’ search at RxISK.org (for 1 to 13 years old) likely represents the Brazilian case, but it is baffling why this case report cannot be found within the FDA’s AERS database. In addition, the latter RxISK search engine yields a report of ovarian enlargement in a search of 1 to 13 years old, which is also not found within the FDA’s AERS database.
In a search of AERS for adult women who experienced oophorectomy post-Lupron, 42 reports were found, and all but three reports were expedited, 15-day reports (which are provided in cases of serious adverse drug reactions). In a search of the “Lupron Depot-PED” AERS, 3 cases of ovarian cyst were reported. It is well known only 1% – 10% of all serious adverse events are ever reported to the FDA – meaning 90-99% of adverse events to Lupron are not reported [see page 7 here].)
Off-Label Use for Gender Dysphoria
In the off-label use of Lupron for ‘pausing puberty’ in the transgender population, it should be understood that Lupron is rarely identified as “Lupron”, but is called a “puberty-blocker”, “hormone blocker”, or “a puberty-suppressing drug”. No doubt this language shift is an attempt to prevent an association with the ‘dreaded Lupron’. It should also be noted that a reproductive biologist has stated ‘puberty suppressing treatment’ “impairs the children’s reproductive capacity” and:
“[s]ome trans boys (i.e. girls) receive puberty-suppressing treatment and never produce mature ovarian follicles … the problem is accentuated with trans girls (i.e. boys) because their spermatozoa are still developing.”
Additional alarming acknowledgments within the transgender population’s off-label use of Lupron are that:
“[p]otential long-term effects can include other abnormalities of hormones, vascular complications and even potential cancer.”
According to UnitedHealthcare policy, “pubertal suppression therapy is considered unsafe in managing children and adolescents with gender identity dysphoria and is, therefore, not covered.” Other insurers do cover treatment of gender dysphoria with Lupron. One Canadian consent form for Lupron treatment of natal females with gender dysphoria identifies a number of risks, and twice repeats the warning that “there may be long-term side effects we do not yet know about”.
In 2015, the NIH awarded $5.7 million for a 5-year multi-center study which
“will be the first in the U.S. to evaluate the long-term outcomes of medical treatment for transgender youth“, seeking data on the “physiological and psychosocial impact, as well as safety, of hormone blockers.”
Reproductive and Developmental Toxicant
Lupron is known as a “recognized reproductive and developmental toxicant“. The manufacturer’s ‘Material Safety Data Sheet’ (MSDS) identifies that Lupron-PED “may impair fertility” and “may damage fertility”. The product label states the effects are “reversible on discontinuation of drug therapy” and “normal pituitary-gonadal function is usually restored within six months after treatment with LUPRON DEPOT-PED is discontinued” (emphasis mine). The label also identifies that rats “demonstrated tubular degeneration in the testes even after a recovery period.” Past product labels state “no clinical studies have been completed in children to assess the full reversibility of fertility suppression”, but in 2013 follow-up data from previous pediatric clinical trials identify that post-study surveys of 20 trial participants found 80% had normal menstrual cycles – which indicates 20% had abnormal menstrual cycles.
Lupron, Endometriosis, and Hypoestrogenism
It is pertinent to address here the findings of an independent analysis by Dr. David Redwine of the raw data from Lupron’s endometriosis clinical trials: this analysis evidenced, among others,
“62.5% of [Lupron Depot 3.75 mg.-treated endometriosis] patients had not regained baseline estrogen levels by one year after stop of study … [indicating] definitive evidence of long-term damage to ovarian function” (see ‘Alarming Facts About Lupron’s Risks’ on pg. 26 here).
In a stark and most troubling contrast, Lupron Depot’s product label states the Lupron-induced hypoestrogenism “is reversible upon discontinuation of therapy”. Lupron’s manufacturer sought and obtained a court seal (see page 6 here) upon its endometriosis clinical trial data (and my attempts to unseal this data were unsuccessful). Without access to this raw data, further independent validation is not possible. To this day, these studies remain in the published literature without any retraction or comment. And cumulatively, as of this writing, these published clinical trials have been cited – as fact – within 23 PubMed Central articles (as recently as 2016), and they have also been cited in three Cochrane Systematic Reviews. The four published studies containing the questionable endometriosis clinical trials’ data are studies “M84-042“, “M86-031“, “M86-039“, and “M92-878“.
The alarming contradiction in data and outcomes (raw endometriosis data showing “62.5% experienced long-term damage to ovarian function” vs. Lupron’s label and published studies’ claim of “reversible upon discontinuation”), as well as the perplexing paralysis on the part of the FDA and medical journals to act on behalf of public health, begs for a high beam investigative spotlight by the media, and medicine. See FDA response which completely ignores the issue of discovered fraudulent data in Lupron’s endometriosis clinical trials, and see 2014 letter to FDA by Lupron Victims Hub which remains unanswered.
Somebody needs to answer these questions. If the FDA is not able or willing to be in charge, then who is the responsible authority? Inaction in this matter is totally unacceptable on multiple levels.
What Does the Future Hold?
Lupron has been administered to children for 30+ years, yet no definitive conclusions about its effects upon reproductive health can be made due to lack of data?
The NIH transgender study, which should include assessment of “hormone blockers” upon the reproductive system, won’t be completed until 2020. But even if study results were available today, would it be claimed the data from the transgender population is not transferable to the precocious puberty population?
If various medical boards can classify Lupron’s use in children with autism as “dangerous, abusive and exploitative”, then Lupron’s use in children (period) is dangerous, abusive, and exploitative.
Share your Story
If you have a Lupron story, please consider sharing it on Hormones Matter.
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
This article was originally published on March 15, 2017.
Photo by Jasmin Egger on Unsplash.






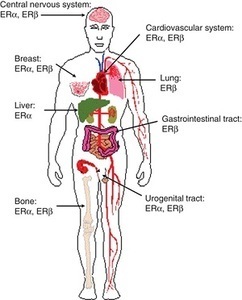 Am I missing some great medical insight that suggests we really don’t need those pesky hormones after all; that all of those hormone receptors located all over the brain and body are there just because? How can a drug like this be used so cavalierly in young women? I don’t have answers for those questions beyond a collective insanity that has permeated medical science where women’s health is concerned. Absent answers, however, what I can begin to provide are data regarding the scope and severity of potential side effects associated with this drug; data gathered from the women themselves, unfiltered by industry bias or potential economic gains. Indeed, I suspect, once the full results of this study are published, industry will be none too happy with me or with our little project. Cue trolls.
Am I missing some great medical insight that suggests we really don’t need those pesky hormones after all; that all of those hormone receptors located all over the brain and body are there just because? How can a drug like this be used so cavalierly in young women? I don’t have answers for those questions beyond a collective insanity that has permeated medical science where women’s health is concerned. Absent answers, however, what I can begin to provide are data regarding the scope and severity of potential side effects associated with this drug; data gathered from the women themselves, unfiltered by industry bias or potential economic gains. Indeed, I suspect, once the full results of this study are published, industry will be none too happy with me or with our little project. Cue trolls.



