My life and health were turned upside down after my unwarranted hysterectomy. I touched on the internal and external anatomy changes in a previous article. I am going to go into more detail here on the effects of hysterectomy on the internal anatomy.
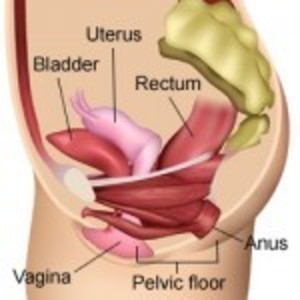
Female Anatomy
The uterus sits in the center of the pelvis held in place by four sets of ligaments. The uterus separates the bladder and the bowel and holds those organs in their rightful positions. Once the ligaments are severed and the uterus removed, the bladder and bowel drop down and, without the uterus to separate them, are now adjacent to each other. The nerves and blood vessels that are severed during hysterectomy may also alter the functions of pelvic organs. This female anatomy video explains the anatomical (and other) effects of hysterectomy.
What Every Woman Wants to Know about Hysterectomy
Pelvic Floor Disorders after Hysterectomy
What do medical studies say about the effects of these anatomical changes on the pelvic floor and organ function?
This 2014 U.S. study concluded that hysterectomy is one risk factor for developing pelvic floor disorders. The others are higher Body Mass Index (BMI) and greater parity. There are a number of studies that came to this same conclusion.
This large 31 year Swedish study concluded that hysterectomy, particularly vaginal hysterectomy, even in women with no vaginal births is associated with pelvic organ prolapse surgery. The number of vaginal births further increases this risk.
According to this large Swedish study, vaginal hysterectomy had a higher risk of surgery for pelvic organ prolapse or stress urinary incontinence than other modes of hysterectomy.
Of course, women who undergo pelvic organ prolapse surgery represent only a subset of those who suffer symptoms of bladder and/or bowel dysfunction.
Bladder Function after Hysterectomy
A number of studies have shown no short-term urinary adverse effects of hysterectomy. However, longer-term follow-up shows an increased risk. This large Swedish study over a 31 year period (1973 to 2003) showed a 2.4-fold risk of urinary stress incontinence surgery in women who had hysterectomies for benign conditions. This Danish study of women aged 40 to 60 years also showed a 2.4-fold risk of stress incontinence in women who had a hysterectomy. A small China study showed a 7.6% rate of pelvic organ prolapse and 67.4% rate of urinary incontinence 6 years post total hysterectomy.
A systematic review of 12 MEDLINE articles that used original data published over a 32 year period (January 1966 to December 1997) “was consistent with increased odds for incontinence in women with hysterectomy….Among women who were 60 years or older, summary odds ratio for urinary incontinence was increased by 60% but odds were not increased for women younger than 60 years.” Another review of this same data consistently found an increased risk of incontinence many years after hysterectomy. However, this study also concluded that “Oral estrogen replacement therapy seems to have little short-term clinical benefit in regard to incontinence and is associated consistently with increased risk of incontinence in women aged 60 years and older in epidemiologic studies.”
The latter statement begs the question “Is the association of oral estrogen and incontinence solely from the oral estrogen or could it be that it’s caused by hysterectomy that prompted the use of estrogen?”
Hysterectomized women of ALL ages were at increased odds for urge (1.9) and bothersome urge (2.6) urinary incontinence (but not stress incontinence) according to this Netherlands study of 1,626 women. This French study of 1,700 women also concluded that hysterectomy increases risk of urge, as well as stress, incontinence regardless of age.
In contrast, this analysis of studies done on urodynamics before and after hysterectomy concluded that “Hysterectomy for benign gynecological conditions does not adversely impact urodynamic outcomes nor does it increase the risk of adverse urinary symptoms and may even improve some urinary function.”
This small study compared incontinence / continence at 1 to 3 years post-hysterectomy and again at 4 to 6 years post-op. Interestingly, some women went from being continent to incontinent while others went from being incontinent to continent.
Why the conflicting results? There are a few things that come into play, the more obvious ones being study design and size as well as the follow-up period. Mostly, the results depend on the reason for the hysterectomy and whether a bladder suspension was done at the same time. Two common reasons for hysterectomy are fibroids and uterine prolapse. Both conditions can cause urinary symptoms such as frequent urination and incontinence. So symptoms may improve after hysterectomy and if the bladder was suspended at the time of hysterectomy (in the case of prolapse), that would also explain improvement.
Bowel Function after Hysterectomy
Some studies show that hysterectomy negatively affects bowel function. While this small and short-term 2004 study (comparing pre-operative to 6 and 12 months post-operative) concluded that vaginal hysterectomy does not increase incontinence or constipation, abdominal hysterectomy may increase risk “for developing mild to moderate anal incontinence postoperatively and this risk is increased by simultaneous bilateral salpingo-oopherectomy.” In contrast, this small 2007 study found that vaginal hysterectomy significantly increased anal incontinence at the three-year point and at one and three years for abdominal hysterectomy. However, there was “no significant rise in constipation symptoms or rectal emptying difficulties in either cohort through the follow-up.”
This contradicts this small case control study that showed significant short-term decreased bowel frequency and increased urinary frequency after hysterectomy. It also contradicts this larger Netherlands retrospective study in which 31% of women reported severe bowel function deterioration and 11% reported moderate bowel changes after hysterectomy. In the control group which consisted of women who underwent laparoscopic cholecystectomy, 9% reported “disturbed bowel function.”
Constipation, straining, lumpier stools, bloating, and feelings of incomplete evacuation were reported by women who had undergone hysterectomy in this small study.
Abdominal hysterectomy is associated with a significant risk of fecal incontinence and rectoanal intussusception according to this small retrospective study.
Post Hysterectomy Fistula
Hysterectomy increases risk of fistula as documented in the below excerpt from this article:
The uterus precludes fistula formation from the sigmoid colon to the urinary bladder.
As well as this excerpt from this article:
The most common types of fistula are colovesical and colovaginal, against which the uterus can act as an important protective factor.
Diverticulitis is a known risk factor for fistula formation. This large study looked at the risk of fistula formation in hysterectomized women with and without diverticulitis using data from women hysterectomized between 1973 and 2003. Women who had a hysterectomy but no diverticulitis had a 4-fold risk of fistula surgery compared to women who did not have a hysterectomy or diverticulitis. Women who had a hysterectomy and diverticulitis had a 25-fold risk of fistula surgery whereas non-hysterectomized women with diverticulitis had a 7-fold risk.
Vaginal Vault Prolapse
The International Continence Society defines vaginal vault prolapse as “descent of the vaginal cuff below a point that is 2 cm less than the total vaginal length above the plane of the hymen.” This Obstetrics and Gynecology International article states that “it is a common complication of vaginal hysterectomy with negative impact on women’s quality of life due to associated urinary, anorectal and sexual dysfunction.” The article cited above explains the mechanism for this common complication in section 2 titled “Anatomic Background.”
Table 3 in section 12 compares vaginal and abdominal corrective surgery outcomes using a 5 year follow-up. Vaginal had significantly higher post-operative incontinence and recurrence rates. The re-operation rate due to recurrence was 33% in the vaginal group versus 16% in the abdominal group.
Surgical mesh is used for many pelvic organ prolapse surgeries. And as shown by the TV ads, surgical mesh has high complication rates. It can cause infection and the mesh can protrude into the vagina leaving sharp edges having obvious negative effects on male partners. And removal of all traces of mesh may be impossible because tissue grows around the mesh.
Women who have not had a hysterectomy and have pelvic organ prolapse may choose to use a pessary instead of undergoing surgery to suspend the uterus (and bladder) or undergo hysterectomy. But a pessary may be difficult to hold in place in women who have had a hysterectomy since the walls of the vagina are no longer supported by the uterus and cervix.
Hysterectomy Consequences
Hysterectomy can have serious consequences on bladder and bowel function and increase risk for future surgeries, but the research is mixed, primarily due to differences in methodology. Pelvic organ prolapse is also a possibility. Important variables that increase or decrease the risk for future problems include the reason for the hysterectomy and pre-operative bladder and bowel function. If endometriosis, fibroid or other conditions compromise or affect bladder and bowel function pre-surgery, then odds are they will be affected post-surgery and whether there is improvement or further damage depends upon a number of factors, including the surgeon’s skill. In contrast, and I think where most women are interested, is whether these problems can arise post-hysterectomy when no such problems existed pre-surgery. The answer is yes, there is an increased risk for both urinary and bowel incontinence post hysterectomy.
Additional Resources
This RadioGraphics article details the pelvic organ sequelae that can be caused by obstetric and gynecologic surgeries and the imaging techniques for diagnosing them.
This Medscape article details the Long-term Effects of Hysterectomy.