This is a story of a mother with undiagnosed vitamin B deficiencies who gave birth to a daughter who was also born with undiagnosed vitamin B deficiencies. In the eyes of conventional doctors and labs, there was not much wrong with us, but we knew that life was harder than it should be. We lived managing debilitating dizziness, daily migraines, fibromyalgia pain, chronic fatigue, allergies, hormonal changes, anxiety, and depression. Until we discovered that we were both hypermobile with histamine issues, hypoglycemic, and had many vitamin B deficiencies. The biggest challenge was for my daughter to start taking thiamine (vitamin B1). Her heart rate was all over the place and she had such a bad paradoxical reaction to thiamine that we believe she had been living with undiagnosed beriberi along with POTS.
Mom’s Health Marked by Asthma, Anxiety, Migraines, and a Difficult Pregnancy
All I remember as a child is being afraid to talk in school even if I knew the answer to a question. I had allergies and could not exercise due to asthma. During college, I had to read over and over the same thing because I could not concentrate. I worked extremely hard because the fear of failure was too much to bear. I started to have hormonal imbalances and missing periods. I successfully finished college and moved away to another state. That is when migraines started. Later, I became pregnant with my first child and started having blood clots. Anxiety and depression would come and go with hormonal changes.
When I was pregnant with my second child, my daughter, I was sick every morning with nausea. After 6 months of pregnancy, I had gained only 6 pounds. Ultrasounds showed that the baby was growing normally, but I was losing weight. At that point, I also could see blood clots on my leg. I was placed on bed rest. By the 8th month, my water broke and my daughter was born. She was jaundiced and placed under UV light for a week. I also stayed in the hospital for a week dehydrated, with blood clots, and with the “baby blues”. We left the hospital after a week, and she had a “normal” development. However, you could see that she was a baby that would not go with anyone, not even the people close to us, indicating some anxiety.
Daughter’s Early Health Issues: Selective Mutism, Asthma, Concentration Issues
When my daughter turned four years old, we moved out of state and that is when she stopped talking outside the house. I later found out that it is called selective mutism, a form of severe social anxiety. She started seeing a school counselor to try to help with her anxiety and self-esteem issues. I brought a girl scout group to my house so that she could start having friends and talk to others in her area of comfort. She also developed asthma and needed nebulizer/albuterol treatments frequently and daily QVAR for prevention. She was given Singulair, but it made her very depressed. Her grades in all classes were all over, from A to D. She would spend the whole time after school trying to complete homework, but she couldn’t. Her teacher told me that she really did not have that much homework. I would ask her to watch the dog eating and to take her outside as soon as the dog finished but she would be wandering around the kitchen and could not pay attention to the dog. Her neurologist gave her Strattera and that helped a little. Her EGG also showed some abnormal activity. The doctor recommended anti-seizure medicine and said that she was probably having mal-petit seizures. I refused medication based on how she reacted to Singulair and because the doctors were using words like “probably” and “just in case”. I kept an eye on her and noticed when she ate ice cream and got asthma. I had her stop sugars and dairy. Soon after that, a teacher called me, excited to tell me that my daughter was talking at school. She also was able to stop all asthma medication except for 2 weeks every year when seasonal allergies would hit. At this point, it had been already four years since she stopped talking outside our house. She started excelling in all classes and we were able to stop Strattera. However, the continuous anxiety remained.
The Teenage Years: Continuous Migraine, More Medications, and No Answers
At 16 years old, she got a cold that turned into asthma with a continuous headache that just would not go away. She started waking up every day with a migraine, depressed with no energy. We had to wait three months to see a pediatric neurologist. Meanwhile, I would take her to my chiropractor early in the morning, give her an Excedrin, and she would go to school whenever she felt better. She began drinking at least 2 cups of coffee every day to help with the pain. Sometimes she would go to school at 11am, sometimes at 1pm. Even if there was just one class left, she would go to school. At this point, she felt that she wouldn’t have a future.
When we finally went to the neurologist, he recommended amitriptyline. I had been on amitriptyline and woke up one day not knowing which year or season was, but I was told that the issue was the high dose given to me (125mg), after decades of it increasing it every year. I agreed as long as it was a low dose. Amitriptyline lessened the continuous headache, but it was not really gone, and she still needed some Excedrin. She started daily aspirin as well. She was just getting by day to day trying to manage her pain and mood and trying to have a normal teenage life.
Increasing Weakness When Outdoors: Untangling Root Causes
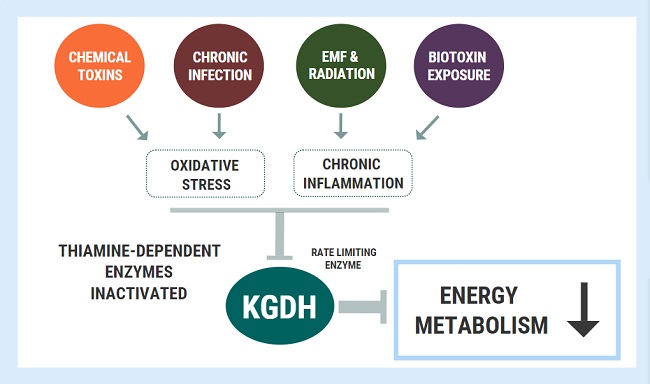
She became very weak whenever we would go to the beach or to a park. We would have to drag her indoors and give her water. On some occasions, she would say that she could not see. Somehow, she successfully managed to graduate from high school. We started seeing functional doctors. We found that she had some variants related to mitochondria dysfunction, but we really didn’t know how to address this. We also found out that she had Hashimoto’s and antibodies against intrinsic factors, which was indicative of pernicious anemia. We knew right there, that she had issues that conventional doctors had missed.
We also did a Dutch test and found that all of her hormones were high. The functional doctors suggested sublingual B12, folinic acid, and a B complex. She said the vitamins made her feel awake for the first time. However, chronic fatigue was still a major struggle for her. Eventually, she had to stop folinic acid because it made her depressed and unmotivated. Meanwhile, she managed her anxiety with herbs, but it was a real struggle. She also continued to have asthma requiring albuterol every fall season. She chose a very challenging career in cell biology with biochemistry. She went through college with many cups of coffee just to control migraines, have energy, and be alert.
Discovering Her POTS Symptoms
The summer of 2019, before her senior year of college, the nurse checked her vitals as part of her new summer internship. The nurse thought the pulse monitor was broken because her heart rate was 120 sitting down. After a few minutes, it went down to 99, so the nurse dismissed it. When she told me that, I started paying attention to her heart rate. We went to her physician and neurologist and in both instances, her heart rate was 100, just sitting down waiting for the doctor. I asked if it was normal, and they said that it was in the upper range but not a concern. I was still concerned and made an appointment with a cardiologist but also bought her an iwatch. She noticed right away how her standing heart rate would be over 100, and by only taking a few steps, her heart rate would go even higher and she would become fatigued and even dizzy. From the heart rate monitor on her iwatch, we could see how quickly her heart rate would climb upon standing and then slow a bit when sitting.
That is when I remember that I have read about POTS and hypermobile people. I remember that when she was a child, the neurologist had said that she was hypermobile, but never said that it could be a problem for her. It just seemed like a fun thing to have. I started asking in health groups and someone mentioned that her medications could also cause high heart rate. I searched and amitriptyline did have that side effect. That is when my daughter showed me that her resting heart rate was in the 90s and it would fluctuate from 29 to 205 without exercising. When we went to the cardiologist and explained all of this, he said that he did not even know how to diagnose POTS because it is rare. He did testing and said that the heart was fine but there was some inefficiency due to some valve leaking but that it usually does not cause symptoms. I asked about amitriptyline and he confirmed that it could raise heart rate. At that point, she stopped amitriptyline and her maximum heart rate was 180 instead of 205.
She went back to her last year of college when Covid hit. She came back home and we could see the lack of energy and how much doing any little thing or stress would crash her for days. Since I needed glutathione for chemical sensitivities, I decided to see if it would help her. Glutathione with co-factors helped her recover, instead of crashing for days, she would recover the next day. That is when she told me that every time she walked to school, she felt that she would pass out. When she gets up in the morning, she ends up lying on the floor because of dizziness. Despite her dizziness, daily muscle pain, daily migraines, and chronic fatigue, she had big dreams. She just kept pushing through day by day, with coffee, herbs, and whatever it took, but she knew that something had to change. She successfully graduated in May, Magna Cum Laude, and she had a couple of months to deal with her health before she would leave to start her graduate studies and research job. That is when I found people that knew about Dr. Marrs’ work and thiamine, and her life finally changed.
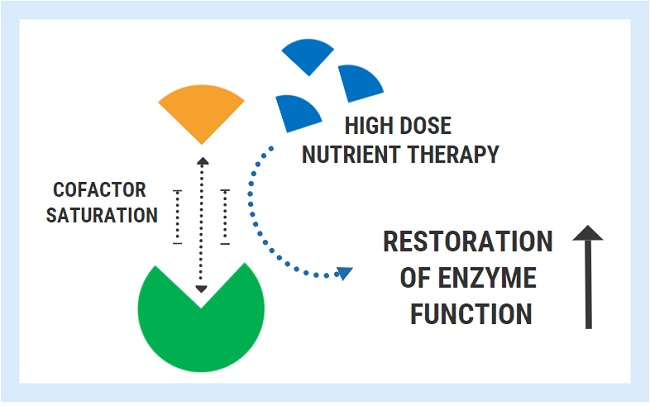
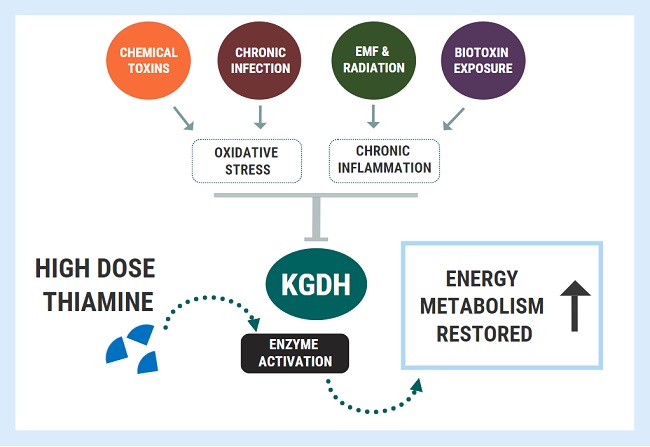
Introducing Thiamine and Other Micronutrients: Navigating the Paradox
A functional doctor recommended magnesium and niacin for her migraines and they significantly helped. This gave the functional doctor the idea to try tocotrienols. High doses of tocotrienols worked better for reducing her migraine pain than amitriptyline and aspirin combined. Then she started taking high doses of B6. This helped her muscle pain and improved her mobility. Despite being hypermobile, easy stretches gave her intense muscle cramps prior to starting B6. Guided by very knowledgeable researchers belonging to Dr. Marrs’ Facebook group, Understanding Mitochondrial Nutrients, we started Allithiamine. The first thing she said was “wait, the sun does not hurt?”. I asked her what she meant. She explained that all her life, being in the sun gave her pain in her eyes and forehead and that she couldn’t understand why people wanted to be outside. No wonder she never wanted to go outside. She also said her migraines were gone. We have waited 4 years to hear that!
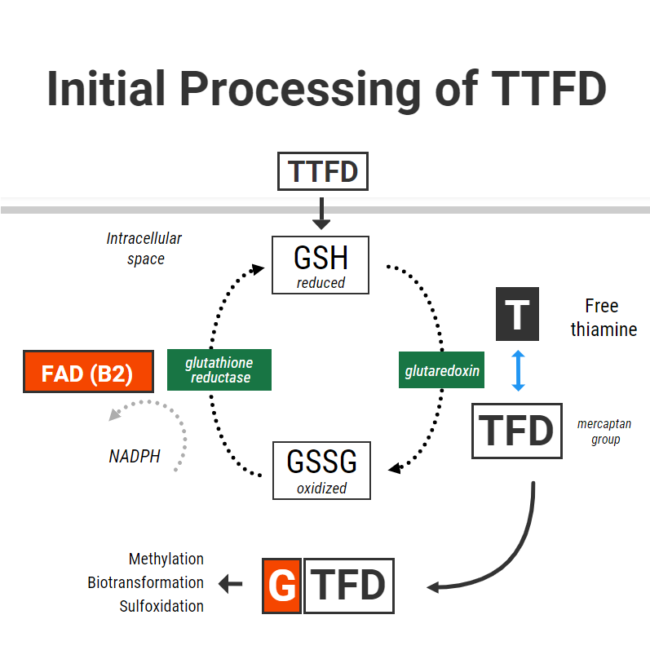
After just a couple of days, she started having a lot of nausea and lower-intensity migraines returned. The researchers knew right away that she needed more potassium. She started to eat apricots, coconut water, or orange juice every time she had nausea and it helped. However, it was happening every hour so we decided to try a different Thiamine. We tried half Lipothiamine and Benfotiamine but she didn’t feel as much benefit and still gave her issues. We went back to 1/10 of Allithiamine. Chatting with the researchers, one asked if she also experienced blinding episodes. Yes! Finally, someone that knew about that! They recommended B2 and we started it. That’s when we discovered that her pain in the sun and dizziness were caused by a B2 deficiency. She continued waking up with crashes needing potassium every hour. She did not sleep that week. The researchers suggested taking cofactors including the rest of the B vitamins, phosphate salts, phospholipids, and beef organs. Beef organs and phospholipids helped with energy and bloating, phosphate salts helped with nausea and irritability.
Then researchers suggested that she needed to stabilize sugars and have more meat. That is when we realized that she had some type of hypoglycemia. We had noticed that she would get very tired and got shaky hands if she didn’t eat. Functional doctors had mentioned that she may have reactive hypoglycemia since she had a fasting glucose of 70. She started having more meat to stabilize her sugars and removed all packaged foods, sugars, grains, and starches. She started having just fresh meat, veggies, rice, beans, nuts, and berries. She felt that she was so much better with beef that she started using it for potassium between meals and bedtime.
She was able to increase allithiamine little by little. She would mix a little bit with orange juice since it tasted so awful. Little by little, she started having fewer crashes and feeling better. It took a month for her to be able to tolerate one capsule of Allithiamine. She was sleeping more but not the whole night. That is when our functional doctor suggested supporting adrenals. That really helped but then she began having stomach pain and nausea after eating beef and developed frequent diarrhea. Chicken always increased her hunger and reduced her energy compared to beef and but now she was afraid of having beef. She stopped all sources of beef and phospholipids.
We consulted a very good functional doctor. She did Nutraeval and confirmed that all her B vitamins were low or deficient and recommended TUDCA and Calcium D Glucarate along with trying lamb and bison first. Both helped in reducing bloating/nausea and she was able to start eating lamb and bison along with reintroducing a minimal amount of carbs. Soon after, she was eating beef again with no pain. After starting TUDCA, her bilirubin levels were normal for the first time in her life. We continued to work with the functional doctor to fix other deficiencies.
Recovery from Multiple Nutrient Deficiencies and the Prospect of a Normal Life
After Allithiamine and vitamin B2, we worked with our functional doctor to balance the remaining B vitamins. She is now able to go out in the sun without bothering her eyes and without passing out. She gained weight after starting the B vitamins and began looking healthier, compared to how skinny and underdeveloped she looked before. She also learned how to manage electrolytes. She sometimes needs more sodium, but other times needs more potassium. She feels sick when electrolytes get out of balance. Although she still had some continuous pressure in her head, she no longer needs any amitriptyline, aspirin, or Excedrin for pain. One thing that remained problematic was folate deficiency. She still became depressed with folinic acid, so she tried methylfolate instead. She felt so unmotivated that preferred not to have it, but she realized that it was key to something that she struggled with all her life: anxiety. She figured that she could have methylfolate every other day, so that she could have less anxiety.
Now, for the first time, she began to have a normal life. She can now exercise daily without dizziness and her heart rate skyrocketing. Her heart rate in general is more normal, doesn’t go down to 29 or up to 205. She had not had any asthma requiring albuterol. She started driving without having to deal with anxiety and panic attacks. She was able to walk to her office without fainting. She now can now live alone dealing with the stress of having a full-time job, graduate classes, cooking her food, and exercise every day! She is not cured completely but for a person that once thought she couldn’t have a future, she is doing pretty darned good!
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, and like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
This article was published originally on July 22, 2021.