Hair loss is a common symptom of thyroid disease. In our research, hair loss, changes in color or luster, and skin changes are regularly reported as one of the first symptoms noticed in an emerging non-allergenic adverse reaction to a medication or vaccine. These symptoms often coincide with unexplained fatigue and muscle pain. Given that many patients who develop the more chronic, multi-symptom medication or vaccine reactions also develop thyroid disease and frequently exhibit signs of mitochondrial damage, I wondered if somehow the hair and skin changes could be early warning signs of diminished mitochondrial functioning. I also wondered if all of these variables were connected. It turns out that not only are they connected, but incredibly interdependent.
What are Mitochondria?
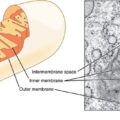
Recall from high school biology, the mitochondria are those bean-shaped organelles inside cells that are responsible for cellular respiration or energy production. Through a variety of pathways, the mitochondria provide fuel for cell survival. In addition to cellular energy production, mitochondria control cell apoptosis (death), calcium, copper, and iron homeostasis, and steroidogenesis. In essence, mitochondria perform the key tasks associated with cell survival, and indeed, human survival. Damage the mitochondria and cellular dysfunction or death will occur. Damage sufficient numbers of mitochondrion and chronic, multi-symptom illness arises.
How to Damage Mitochondria
Mitochondria are remarkably resilient given the proper nutrients, but without those nutrients, they can be highly susceptible to damage. Mitochondrial damage can be inherited via mutations in maternal DNA (mtDNA) or nuclear DNA and present at birth or remain latent until triggered later in life, as in the case of mitochondrial endocrinopathies. Mitochondria are also susceptible to epigenetic changes, which can be heritable and acquired and remain latent until triggered. Finally, mitochondrial impairment can derive from pharmaceutical or environmental exposures and nutrient or cofactor deficits. The sheer number of mechanisms that can influence mitochondrial functioning and heritability make diagnosing and predicting mitochondrial dysfunction difficult at best, particularly acquired or functional mitochondriopathies that are not evident from genetic or epigenetic testing. It is precisely those acquired mitochondriopathies, particularly those seemingly triggered by pharmaceutical reactions, that we are most interested in here at Hormones Matter. Indeed, acquired mitochondrial damage represents a nascent and emerging field in medicine, particularly in toxicology, as many drugs and vaccines damage mitochondrial functioning both directly and indirectly.
What Mitochondrial Damage Looks Like
Mitochondrial damage presents in a highly diverse, multi-organ, multi-symptom manner. On the surface, patients with mitochondrial dysfunction will appear to have multiple, unconnected diagnoses, from gastrointestinal distress to cognitive deficits, from cardiac arrhythmias to multiple sclerosis-like symptoms, and everything in between and beyond. According to Dr. Richard Boles, an expert on mitochondrial dysfunction:
“Mitochondrial dysfunction doesn’t really cause anything, what it does is predisposes towards seemingly everything. It’s one of many risk factors in multifactorial disease. It can predispose towards epilepsy, chronic fatigue, and even autism, but it doesn’t do it alone. It does it in combination with other factors, which is why in a family with a single mutation going through the family, everyone in the family is affected in a different way. Because it predisposes for disease throughout the entire system.”
This is partially because the human body contains over a billion mitochondria which are essential to cellular functioning in every cell of the body. Where the dysfunction emerges is dependent upon where the impaired mitochondria reside, by what mechanism the mitochondria are damaged, and how intervening variables, such as overall health, nutrition, and environment come into play. Given the mitochondrion’s role in energy production, highly energy-dependent tissues such as the brain, the heart, the liver, and even muscles, are most susceptible to direct mitochondrial damage. And considering the mitochondrion’s role in cellular energetics, fatigue is almost always present with mitochondrial dysfunction.
Hormone Synthesis and Mitochondrial Functioning
Adding yet another layer of complexity, mitochondria also control steroid production in the adrenal glands, ovaries, testes, and thyroid. Any impairment of mitochondrial functioning can have a significant influence on hormone production and regulation. Since hormones, like the mitochondria, also impact all facets of biological homeostasis, energy, and metabolism, damage to endocrine mitochondria can represent a double-hit and begin a cascade of endocrine ill-effects that are difficult to control. This is particularly true of the thyroid gland.
Thyroid Hormones and Mitochondrial Functioning
The cells within the thyroid gland are dependent upon proper mitochondrial functioning to maintain health and proper mitochondrial functioning is dependent upon thyroid hormones to manage cellular energy production. This reciprocal and interdependent relationship makes the thyroid especially susceptible to a mitochondrial spiral. Both thyroid and mitochondrial damage have been observed in our medication and vaccine adverse reaction populations.
Thyroid hormones regulate mitochondrial functioning. Triiodothyronine (T3) in particular is considered one of the major regulators of mitochondrial activity stimulating mitochondrial biogenesis (the birth of new mitochondria) both directly (genomic), indirectly (non-genomic), and epigenetically.
T3 is responsible for increasing cellular heat production and oxygen consumption, core activities of mitochondrial metabolism. In hypothyroid states, heat and oxygen are reduced, whereas, in hyperthyroid states, the two are increased. Here the intracellular patterns of heat and energy production correspond to the clinical symptoms of hypo- and hyperthyroid states. Other thyroid hormones along the hypothalamus – pituitary – thyroid axis (HPT) and the other iodothyronines within the thyroid hormone metabolic pathway influence mitochondrial functioning. Remove or reduce the presence of the thyroid hormones and mitochondria produce less energy and eventually die. With them, the cells in which they reside die too. Conversely, as mitochondria within the thyroid become less efficient, smaller concentrations of thyroid hormones are produced. With reduced thyroid hormones, mitochondrial efficiency continues to decline and so on, and so on.
Hair Follicles: Mini – HPTs
German researchers recently identified multiple mechanisms by which human hair follicles are responsive to thyroid hormones. Their research showed that human skin and hair follicles possess an equivalent peripheral HPT axis with all of the corresponding hormones such as the central HPT. It turns out that hair follicle mitochondria are differentially responsive to each of the thyroid hormones along that axis and are responsive to other iodothyronines not typically considered bioactive, such as diiodothyronine (T2).
An interesting finding, related specifically to the hair follicle, and perhaps other mitochondria, thyroid hormones were protective against reactive oxygen species (ROS) production via multiple mechanisms. ROS, also called free radicals or oxidants, are natural by-products of oxygen (energy) metabolism important to a number of basic cell and life processes, like signaling and the defense against pathogens, but ROS levels must be kept in strict balance. Too much or not enough ROS and health goes awry. In the case of adverse fluoroquinolone reactions, increased ROS production is implicated. According to the hair-follicle study, thyroid hormones protect against ROS production and regulate the enzymes that scavenge for and eliminate free radicals – our own internal antioxidants. If this function is conserved throughout the body, it provides one more reason to investigate and appropriately manage thyroid damage in medication adverse reactions.
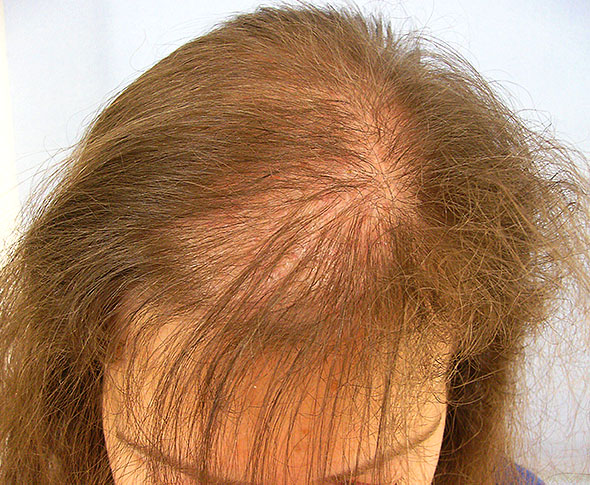
Hair Loss and Mitochondrial Damage
Skin and hair follicles are dense with mitochondria and highly regulated by thyroid hormones such that the mechanism for hair loss in some individuals can be attributed to either diminished thyroid hormones and/or damaged mitochondria. Since the relationship between thyroid hormones and mitochondria is reciprocal, it is difficult to tell which impairment comes first. However, given what we know about hair growth cycles and what we know about thyroid hormones and mitochondrial functioning, it is possible to speculate and backdate a chemical insult precipitating sudden and unexplained hair loss. For more incipient reactions, it is a bit more difficult. Regardless, however, it appears that unexplained hair loss is a sign of poor mitochondrial functioning.
Hair growth occurs in phases. The anagen phase is the growth cycle where hair follicles grow about 1 cm per day for 28 days. This growth phase lasts for 2-7 years. The exact time frame is genetically, or more specifically, epigenetically determined by factors associated with the health of the maternal grandmother. After the anagen phase, the hair follicles reach a transitional, quiescent period lasting approximately 2-3 weeks. This is then followed by the telogen phase where hair begins to fall out. At any given time, up to 90% of hair follicles are in the anagen or growth phase while the remaining follicles are either catagen (10-14%) or telogen phases (1-2%).
Chemo Induced Hair Loss: Answers in the Mitochondria?
With chemotherapy, hair loss begins 2-4 weeks after treatment begins and although multiple mechanisms have been investigated, none have been able to explain or treat effectively chemo-induced hair loss. I would suspect that given the time frame, the toxic insult of chemotherapy, the role of mitochondria in hair growth, and the connection to thyroid damage, that chemo-induced alopecia is representative of mitochondrial damage. The ability to maintain hair growth during chemo may be related to supporting mitochondrial and/or thyroid health.
In the case of other presumed less toxic or at least less directly toxic chemical insults such as medication or vaccine adverse reactions, the initial loss of hair that begins either in the weeks preceding the full onslaught of symptoms or coincident with those symptoms, marks a decline in mitochondrial functioning and likely an impending decline in thyroid functioning.
Hair Loss: A Reallocation of Mitochondrial Resources
Considering, that hair generation is an energy (read mitochondrial) intense process, sudden hair loss could be an early marker that mitochondrial resources are limited and being reallocated towards more critical operations like brain and heart functioning. When the components for proper mitochondrial functioning are absent, be it the thyroid hormones or the co-factors necessary for cellular energy (ATP) production, the first wave of resource allocation might be to cease non-essential activities. The non-essential activities would include hair growth (and wakefulness in general – read Medication and Vaccine Adverse Reactions and the Orexin – Hypocretin Neurons). Sudden or unexplained hair loss could indicate mitochondrial impairment. Backdate the hair loss 2-4 weeks and an illness, a medication, vaccine, or environmental exposure could be the culprit. Whatever the cause, the thyroid and mitochondrial health should be considered and treatment initiated accordingly because if the disease process continues, the symptoms will expand beyond the hair, potentially to every tissue and organ in the body. Concurrently, investigate and amend nutritional status. Mitochondrial functioning is critically dependent on proper nutrients. Deficits in important nutrients, like thiamine, can have severe repercussions.
Feed your thyroid. Feed your mitochondria.
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, and like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
This article was first posted on Hormones Matter in May of 2014.