Downward Spiral, Upward Hope, Asking Advice
The year 2020 marked a big change for a lot of people. For me, it meant a downward spiral into intense fatigue, brain fog, and heart palpitations. Healing came in increments over the next three years until I found thiamine, which expedited my healing in six months. Now, I am considering pregnancy, but I need your advice. How do I navigate taking megadoses of supplements while growing a baby? How do I know when my healing journey is “complete,” and does that mean my supplement regimen ought to change? Any and all comments are welcome!
How It Started
It was early 2020 when I returned from a vacation from Thailand and got a stomach bug somewhere along the way home. I rested in bed a couple of days and mostly recovered, but had a lingering burning sensation in my stomach for the next month or so. I then noticed my stool started to smell strange and I experienced bloating after some meals. I went to the gastroenterologist, and within a month they had done an endoscopy and discovered erosive gastritis. I was put on a proton-pump inhibitor (PPI) and sucralfate to coat my stomach.
Two weeks on these medications and I felt immense brain fog and extreme fatigue, so much so that I felt like I would fall over in my chair at work. The fatigue hit me like a ton of bricks– I slept throughout the night and forced myself to take naps, and nothing helped shake the overwhelming fatigue. I took a few weeks off of work and tried to rehab myself at home, eating as much healthy food as possible (I was tracking 3,000 calories a day, which I felt I must need to get healthy again). I tracked all of my nutrients in an app and made sure I hit (and exceeded) the RDA for every nutrient (with the help of supplements). Still, things were not improving much, and I couldn’t even walk one stretch of the block without being utterly exhausted. It was during this time off of work where I felt so helpless and drained in every sense that I remember thinking, “this is what the beginning of dying feels like.” It scared me. But I honestly did not know what to do or where to turn.

I knew the medications were not making me healthier (even if they made my stomach feel better), so I went off of them cold-turkey. The burning in my stomach became quite severe due to the rebound effect of getting off a PPI, but I pushed through, knowing that I needed my body to heal on its own.
The next three years brought incremental improvements, but still much suffering. Intense brain fog, insatiable fatigue that heightened post-exertion, and dysautonomic symptoms plagued me daily. I was waiting for a big break that seemed like it might never come. Little did I know, my time was coming in the spring of 2023.
How It Really Started
It would be easy to blame a stomach bug for all of my problems, but I now know that my nutrient stores have been taxed and depleted over many instances in my life. Here is a snippet of what led me to the crash:
– Childhood: Ear infections (antibiotics), chronic stomach aches, sugar consumption
– Adolescence: Traumatic brain injury (brain sheer, 3 days coma), mononucleosis, asthma
– Young adulthood: chronic UTIs (i.e., chronic antibiotics (including 3 separate Bactrim prescriptions and anti-fungals (fluconazole) afterward), several deaths in the close family (emotionally taxing), monthly naproxen for menstrual cramps, developed gluten sensitivity, shortness of breath (air hungry).
The stomach bug was simply the straw that broke the camel’s back. All of the stressors in my life (physical, emotional, etc.) depleted my body until it couldn’t retain a guise of “healthy” anymore.
My First (Unknowing) Megadose
Throughout the entirety of 2020, I experienced bloating and IBS symptoms. I managed the symptoms well enough with a low FODMAP diet, but one tiny piece of garlic, onion, etc. and I was ruined. I knew I wasn’t healed with this diet, but I didn’t really know how to heal, especially hearing that IBS is something you have to live with for the rest of your life. This scared me, but I wanted to see what answers may be out there.
I came across a study that claimed that the vast majority of participants taking a multivitamin, B-100 complex, and vitamin D3 were cured of their IBS within three months. It seemed like a miraculous and promising study, so I decided to try it myself. Lo and behold, around the three month mark, I was able to incorporate high FODMAP foods without experiencing bloating (it took a stretch of a few weeks to fully incorporate these foods as my body was adjusting).
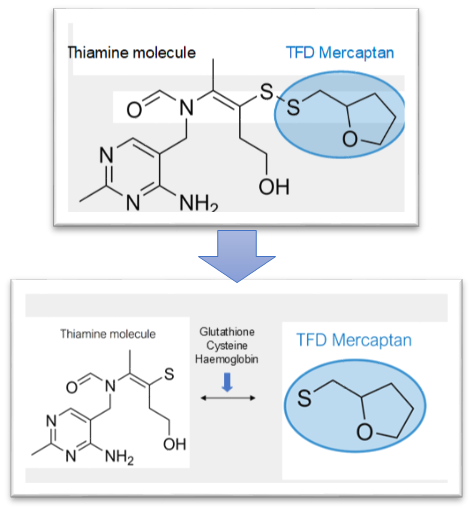
Back then, I thought it was the vitamin B5 that was responsible for ridding me of bloating symptoms. Vitamin B5 is closely linked to gut health. Looking back now, I have a strong notion that I was helped due to the thiamine content in the B-100 + multivitamin. I megadosed without knowing it. And unfortunately, after about 4 months, I stopped taking the B-100.
My Second (Reluctant) Megadose
I visited a naturopath in the spring of 2021 to try to get more answers. I still had brain fog and fatigue, and had also developed a regular heart palpitation every ~15 minutes, which coincidentally happened after my second round of a certain vaccine. The naturopath prescribed many supplements, one of which was 150 mg of iron per day. I was shocked by this and thought that was wayyyy too much and was scared I would get iron overload, but he assured me that with my ferritin levels at a 9, it was desperately needed.
Within a week of supplementing with iron, I felt a big boost in energy and felt I had found the answer that I had been waiting for. While it did help, I reached a threshold of improvement that did not change despite continued supplementation with iron for over 1.5 years. The iron supplements did help with my heart palpitations, but I still had brain fog and fatigue. On a scale of 1-10, with 1 being my lowest point in the summer of 2020, iron brought me to about a 4.
My Third (Homecoming) Megadose
So time went on and I tried every supplement under the sun. I focused on vitamins and mitochondrial nutrients such as L-carnitine, alpha lipoic acid, CoQ10, and others, and I was able to live a life that looked kind of normal. But it didn’t feel normal. I was obsessed with finding the answer(s) to this dark cloud that had been engulfing me the past few years.
Until one day, just six months ago, in late April of 2023, a recommended video popped up on my YouTube homepage that changed my life. The video was from a smart lad named Elliot Overton talking about thiamine deficiency.
You probably know how the story goes.
I started with benfotiamine, because I could get it at the store, while I waited for my TTFD to arrive in the mail. I kept trying to press how much I could tolerate without too much headache/fatigue/brain fog, and I honestly can’t remember if I noticed an improvement in those first few days. Once my TTFD arrived though, within two days of supplementing I felt a rushing wave of beautiful relief come over me.
Finally. Finally! My answer had come. I wasn’t immediately better, but I knew improvement was on its way. It wasn’t long before I came across Hormones Matter, which brought me so much useful information! I began sleeping better. My dreams were more vivid. I was able to sweat more easily, something I didn’t know I had lost until it returned. The volume was turned down on my anxiety and breathing deeply was easier.
It took some adjusting and playing around with dosing to find out what would help me. At first, I could only consistently tolerate one 50 mg TTFD pill every-other day, or I would get a racing heart and worsened fatigue. I also noticed that after about a week of taking TTFD, I would start to feel drained, as if it wasn’t giving me that feeling of relief anymore. So what worked for me was to cycle TTFD, thiamine HCL, and sulbutiamine for one week each. That kept my feelings of “relief” heightened. I pretty much abandoned benfotiamine because, well, I had other stuff that was working and I didn’t want to change my routine.
Within about a month, I was able to take one TTFD per day. As time went on, I kept bumping up all of my doses for each type of thiamine. I would basically take a day to test how much I could handle, then try to sustain that higher new dose. By the end of July, I was taking 5-6 TTFD and 10-ish thiamine HCL (100mg each). I am not exactly sure with the doses. I believe I only made it up to 400 mg of sulbutiamine. At a certain point mid-summer, I dropped the sulbutiamine because it seemed to be making me feel depressed, even though it helped when I first began taking it. I also dropped the thiamine HCL. I felt that TTFD was more powerful and so I stuck with it. I no longer experienced a drop in “relief” symptoms and was able to take TTFD only without any adverse effects.
Somewhere between then and now I have worked myself up to 12-14 TTFD per day (600-700 mg). I have very little brain fog or fatigue and can work out without being drained the next several days. I feel pretty darn good most of the time. Of course, there are ebbs and flows, but overall, I am doing well.
In addition to the thiamine, I have been taking lots of support nutrients too, such as magnesium, multivitamin/B complex, selenium, molybdenum. Another major helper for energy has been 10-15 grams of creatine monohydrate per day. I eat a whole-foods diet with no added sugars.
My Fourth (Aha!) Megadose
Recently, I came across information by Linus Pauling, a Nobel Prize and Peace-Prize winner who championed high-dose vitamin C therapy for minor and major illnesses. I caught a cold around this same time, started high-dose vitamin C therapy, and was absolutely sold with the idea, as none of my symptoms really developed into much at all. While I’m not convinced of taking megadoses of vitamin C every single day, I am certain it is helpful during times of sickness.
Then I read about Orthomolecular Medicine, which uses high-dose vitamins for treating diseases (chronic, communicable, genetic), and it all made sense! I felt as though I had uncovered a secret to the world! I wouldn’t have believed it had I not experienced the “miracles” of megadosing first-hand, but now I realize that most, if not all, diseases can be treated with the right dose of specific nutrients for the right amount of time. I also realize that those doses are higher doses than what we think! And higher still! Yeah– even higher. And longer– yes, keep taking them. I don’t mean to oversimplify people’s illnesses, but rather to illustrate the power of high-dose vitamin therapy.
Then Versus Now
My healing journey is not quite over. I have tested positive for antinuclear antibodies since 2020, and my latest test (October 24, 2023) still tested positive (qualitative only). Finding out these results was a little disheartening, as I really thought my results would be negative. I have had less energy and some mild dysautonomic symptoms since receiving those results, which either means a) the power of suggestion has really gotten to me or b) I switched to thiamine HCL around the same time and it is not as effective as TTFD. I am leaning towards the latter, but I wanted to give HCL more of a shot because the amount of TTFD I’m taking per day is getting expensive! And as a more recent update, the last two days I’ve tried Benfotiamine, which I have been very pleased with— my energy seems to be much better than with thiamine HCL.
I also just started alpha-GPC as a new supplement.
Here is some physical evidence that I am healing:
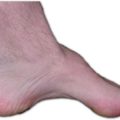
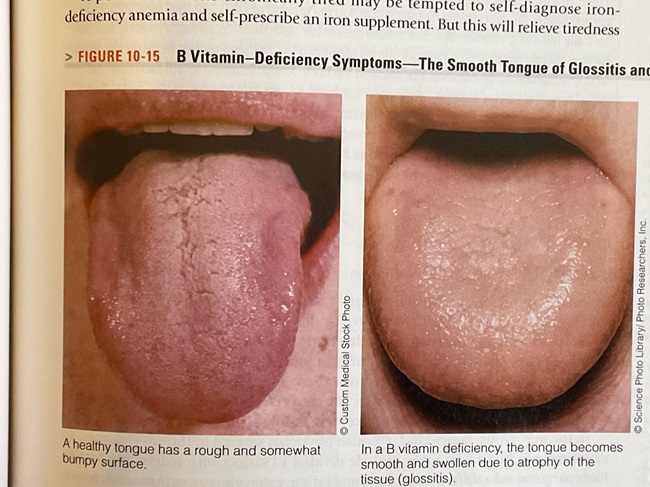
In one of my textbooks, I found that a B-vitamin deficiency (doesn’t say which B vitamin) causes a smooth tongue.
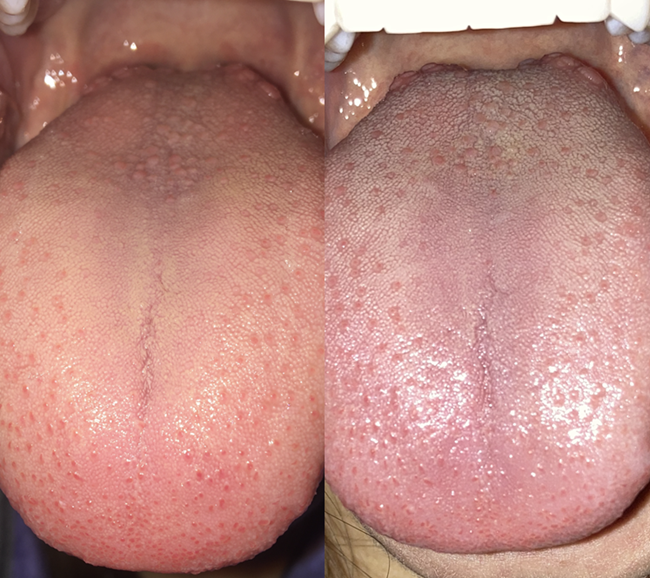
I took a picture of my tongue in October 2020, and the second picture in October 2023. Notice the more prominent fuzzy (white/gray) projections in the second picture. These projections are quite blunted in the first picture.
Hope for The Future
My husband and I are excited about the possibility of getting pregnant, especially now that I am feeling so much better. Having a child has been a long-time dream of mine, and while I was struggling with my health, I wasn’t sure if that dream could come to fruition. So now being in the place I’m in, I’m thrilled that we can think about having a child. I’ve had to tap the brakes on my excitement, because I don’t want to potentially cause any harm to a growing baby due to my megadosing of thiamine. So, I have a couple of questions.
Asking Advice:
- Does anyone have any research, personal, or hearsay information regarding the safety of megadose thiamine during pregnancy? If so, did the type matter (TTFD, thiamine HCL, Benfotiamine)?
- What is the maximum dose you reached for TTFD/thiamine HCL/Benfotiamine?
- Have any of you had any experience with weaning off of thiamine or stopping cold-turkey? I have gone a few days here and there without supplementing with no issue, but not longer than that. If so, was your health maintained, or was there a maintenance dose that sustained you?
- How did you know it was time to stop/decrease thiamine (if at all)?
Closing Thoughts
I just want to extend my heartfelt empathy for all of you who may be experiencing health struggles. Before these past few years, I sometimes had the arrogant thought that people could just be healthy if they avoided sugar and exercised. I thought their health struggles were their “fault”, to an extent, but I now recognize the complexity of health and the desperation in trying to find it once it is lost. I understand what suffering is and the feeling that there is no escape. I understand the feeling that no one truly knows what you are going through, even though they extend love and patience with you. I get it, and it sucks so much that this has to be a part of the human experience—but I have also experienced hope. A real hope. A hope that delivers what it promised. I could not have known even a day before taking thiamine that my time of deliverance had come. So please do not give up hope. Your day is coming.
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
Photo by Tim Mossholder on Unsplash.