Hello, I am a 33 year old male who has been experiencing a vast range of symptoms over four years including progressively worsening fatigue, brain fog, muscle weakness, body pain and erectile dysfunction. Only a few months ago, I discovered that there could be a relevance of thiamine in all of my issues. All of my health problems worsened at the age of 29 years old. More context can be found below.
Childhood Problems
I had problems with:
- Concentration and focus
- Emotionally down
- Prone to common cold (infection)
- Unfortunately I was put on antibiotics more often every few months as a kid (amoxicillin)
- Brain fog
- My brain is slow at processing things, there is always a latency associated with me to perceive things.
Health Journey
My health journey is complex. In the sections below I have tried my best to capture the sequence of events and diagnostic data we have so far. I began developing fatigue, not feeling refreshed even after sleep in my early 20’s but I continued pushing myself – not knowing how to address this. Irrespective of these limitations, I was an active adult – I was working out regularly, lean and athletic. My food habits were clean i.e., no processed foods, no alcohol, but majority of my calories were from carbs. My macro mix was approximately 50% carbs, 25% fat, 25% protein. Being a South Asian, white rice is part of my staple diet.
Things started to go really bad around 29 years of age when my fatigue, brain fog, muscle weakness, body pain worsened and nocturnal erections started to degrade. I was not able to obtain erection without Viagra. I started my health journey to fix my erectile issues. Because of my appearance (lean and athletic) all the doctors refused to even work with me, saying the issues were psychological. I found an alternative doctor who ordered blood work and we found few biomarkers that were off.
- Very low Vitamin-D3 – 12.8
- Low Platelets – 60,000 – 80,000 x10E3/uL
- Subclinical Hypothyroidism (Elevated TSH – 9.5 uIU/mL and Reverse T3 – 33.8 ng/dl)
- Testosterone was low for my age but not below reference range – 525 ng/dl
Unfortunately, this is the first time I had a comprehensive health checkup, so I don’t have any previous data to compare against. Since I was in the Pacific Northwest area where there is not much sun, I was living with low D3 for years. I worked with a hematologist to ensure low platelets were not as a result of any major illness. I started addressing my thyroid using levothyroxine and low vitamin-D3 with a vitamin-D3 supplement.
Subclinical Hypothyroid and Low D3
We developed a plan to address vitamin-D3 deficiency via supplements and also thyroid via levothyroxine. We started with 50 mcg of levothyroxine, which improved some of my symptoms slightly. The fatigue, brain fog and erectile issues improved somewhat. Unfortunately this was short lived and after ~4-6 weeks, my health started deteriorating again. Since I saw initial progress with thyroid, the doctors assumed my health issues were related to thyroid and started treating me with different thyroid formulations, different forms etc., to improve thyroid function.
After looking at my thyroid labs, doctors always mention that my thyroid hormones – FreeT3, FreeT4 were good but my reverseT3 and TSH were always elevated.
Neuropathy and Disc Herniation
In the end of 2020, I began developing burning pain in my lower back, which eventually started flowing to both my feet. MRI confirmed that disc herniation in my L5-S1 layer impacted S1 nerve root. I also took EMG that confirmed there is mild impact on S1 nerve root. The burning pain coincided with worsening erectile dysfunction. I was no longer responding to Viagra and I was in immense burning pain. After a few months of intense pain, the pain has begun to recede, but I am still experiencing a constant burning sensation, except when I sleep.
Disc herniation or burning pain was not as a result of any incident. It seemed to develop gradually, like everything else. A straight leg test or no movement worsened it immediately. One neurologist had an alternate theory that burning pain was not coming from disc herniation but because my D3 was low for a long time my microbiome was affected. Since gut bacteria synthesize B vitamins, she suspected that I was deficient. Her theory was I was deficient in vitamin-B5, which was resulting in burning pain and sleep issues. It is also possible that burning pain is caused by thiamine deficiency. I talk about this in the thiamine supplementation section below.
Neurological Issues
During this time period when I developed burning pain, I was also struggling with temperature regulation issues. When I moved from outside ~90f to inside ~70f, I would get chills. I was feeling cold most of the time, cold hands and feet, and sweating profusely. I used to get pins and needles randomly when out in the sun or while walking.
Gastrointestinal Issues
When I developed burning pain, I also started experiencing bad constipation. I was not able to empty my bowel at all. I had to take herbal laxatives every day for my bowel movement. I have also been experiencing bloating, seeing undigested foods in stool, chronic bad breath – potentially from SIBO. In the last three years, I have lost more than 20 pounds. I look more lean and weak at this point.
Sleep Issues
It has been years since I woke up feeling refreshed. I rarely dream. I have noticed that I am able to easily fall asleep and stay asleep most of the time but my sleep quality is bad, especially the later half of the sleep where REM sleep occurs.
Erectile Dysfunction
I have no nocturnal erections at this point and have not had any over the last several years. I still rely on Viagra and am now taking more than 100 mg, which is the max dosage of Viagra. On some days I don’t respond to Viagra as well. All other obvious issues associated with erectile dysfunction were ruled out including hypertension, heart issues, and hormonal issues. Essentially, I am a ‘healthy’ individual suffering from erectile dysfunction. With all of the other issues, am I really healthy? I don’t think so, but the doctors do.
Toxins and Micronutrient Deficiencies
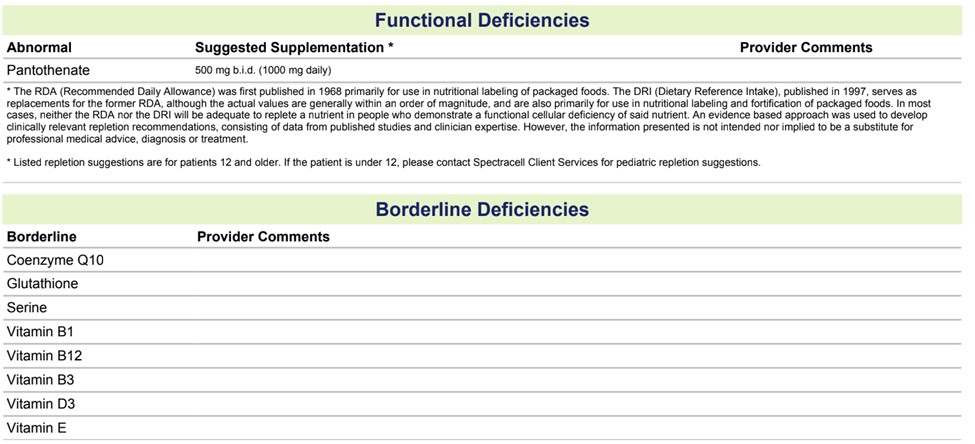
One of the theories of a doctor who evaluated me was that I was exposed to some toxins. Testing revealed that I had high levels of ochratoxin A, a mycotoxin, which is usually from aspergillus but may be impacted by glyphosate exposures. Based on my blood and urine markers, they confirmed that my detox pathways were impaired and in need of more B-vitamins. I also did a Spectracell testing, which looks at the vitamins and minerals in the cell level, and it did show a deficiency in vitamin-B5, and borderline deficiencies in few other vitamins, which supplementing with a multi-vitamin didn’t appear help.
Thiamine (Benfotiamine) Supplementation
I began supplementing with Life Extension – 250mg of Benfotiamine and many things happened.
- My sleep quality improved and I felt slightly refreshed the next morning.
- I started getting partial nocturnal erections.
- I started responding to the same dosage of Viagra much better than before taking Benfotiamine.
- Better energy and mood.
- Burning pain in my feet reduced greatly.
The problem, from second day onwards my sleep quality fell apart. I was easily able to fall asleep but was not able to sleep for more than ~5-6 hours and my REM + Deep sleep was less than 90 minutes.
I increased electrolytes
- Potassium
- Add 1 litre of coconut water
- Added 1 teaspoon of cream of tartar
- Magnesium
- Increased from 250 mg to 375 mg – I am taking Magnesium Malate
This improved my sleep quality slightly, but I still struggled. I couldn’t sustain taking Benfotiamine at the same dosage for a long time. So I had to stop.
Current State
Supplements I take currently:
- Vitamin B12 – 1000 mcg
- Vitamin D3 – 10,000 IU
- Magnesium Malate – 375 mg
- Creatine (~3 grams)
- Athletic Greens (Multi Vitamins)
I am still suffering with all the issues mentioned above and struggling to incorporate thiamine. How should I proceed here?
- Should I try small dosages of TTFD and proceed from there? What cofactors to incorporate?
- Should I work with doctors and take thiamine injections or incorporate IV?
- Should I try Myer’s IV – which contains below formula once a week for few weeks to see if I can experience any improvement to validate this theory
- 5 mL of magnesium chloride hexahydrate (20%)
- 3 mL of calcium gluconate (10%)
- 1 mL of hydroxocobalamin (1,000 μ/mL)
- 1 mL of pyridoxine hydrochloride (100 mg/mL)
- 1 mL of dexpanthenol (250 mg/mL)
- 5 mL of vitamin C (500 mg/mL)
- 20 mL of sterile water
- 1 mL of B-complex 100 containing:
- 100 mg of thiamine HCl
- 2 mg of riboflavin
- 2 mg of pyridoxine HCl
- 2 mg of panthenol
- 100 mg of niacinamide
- 2% benzyl alcohol
I have been very determined to get myself out of these conditions. Any help or guidance here will be much appreciated?
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, and like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.