A few weeks back we published a case story of young man who developed serious cardiac and neurological symptoms after beginning a course of two antibiotics, Bactrim and Keflex. His symptoms immediately reminded me of beriberi induced by an acute thiamine deficiency. After some digging, I found that at least one of the antibiotics, Bactrim, likely does indeed induce thiamine deficiency by potently blocking both thiamine transporters. This had been only recently and accidently discovered and is clearly not common knowledge. What is common knowledge, however, is that Bactrim is also an anti-folate. This was by design, as blocking bacterial folate metabolism makes for a potent antibacterial, which also just so happens, makes it a viable chemotherapeutic. While it is impossible to rule out the Keflex in his sudden illnesses, as this drug too carries potentially serious side effects, or the combination of the two drugs, I would like to focus on Bactrim for this post, and specifically, its anti-folate and anti-thiamine properties. I believe these aspects of the drug may underlie many its adverse reactions in addition to, and perhaps compounding, those associated with its propensity to induce hyperkalemia.
A Medical Workhorse with a History of Adverse Events
Bactrim, a combination of two antibiotics, trimethoprim and sulfamethoxazole, was first approved in 1968 and 1973 in Canada and the US, respectively and has become a mainstay in pharmaceutical medicine. It is prescribed for everything from acne and UTIs to prophylactic treatments associated with HIV. On average, there have been between 6-7 million prescriptions of this drug written annually from 2007-2017, except for in 2015, where almost 12 million prescriptions were written.
It is one of those antibiotics, that because it has been around for so long, is considered safe and benign. In reality, however, there are a number of serious side effects associated with it including:
- Aseptic meningitis – inflammation of the meninges (outer layer of the brain) absent bacterial involvement.
- Hemolytic anemia – the destruction of red blood cells more quickly than they can be made particularly in folks with glucose-6-phosphate dehydrogenase (G6PD) deficiency. One study of hospitalized patients found 6 per 100,000 prescriptions resulted in hemolytic anemia.
- Methemoglobinemia – decreased blood oxygen saturation via increase methemoglobin.
- Delirium, gait disturbances and other CNS symptoms – owing to the ease in which it crosses the blood brain barrier. In one study of elderly patients, cerebral spinal fluid concentrations were ~50% of that in serum.
- Stephens – Johnson Syndrome and toxic epidermal necrosis – a potentially life-threatening reaction where the lining of the skin and mucous membranes blisters and necrolyses.
- Acute respiratory distress syndrome (ARDS), requiring mechanical ventilation, sometimes leading to organ transplant, often with poor outcomes, including death. This is particularly troubling, as it has been identified in children.
- Renal disorders including: hyperkalemia and hyponatremia, and an acute interstitial nephritis, but also, hypertension and cardiac events, sometimes fatal. In immunocompromised patients, the rate of hyperkalemia with Bactrim hovers around 20%. Case studies suggest Bactrim induced hyperkalemia in non-immunocompromised patients as well, though its incidence is neither studied nor reported. In patients who also use spironolactone, a potassium sparing diuretic known for inducing hyperkalemia that is widely prescribed for hypertension, heart failure and for acne, but also as birth control (drospirenone is a spironolactone analog used in the oral contraceptive brands such as Yas, Yasmin and others), the risk of sudden death doubles. Although, the spironolactone + Bactrim > sudden death relationship is studied typically only in the elderly, both drugs are increasingly common in adolescents and young women and may account for a good percentage of the adverse events in these populations that are considered idiopathic. Similarly, the popular angiotensin enzyme inhibitors (ACE) and angiotensin II receptor blockers (ARB) used for hypertension, when combined with Bactrim carry 7-fold increased risk of potentially deadly interactions involving hyperkalemia.
A review published in 2011, identified 925 papers on the adverse effects of trimethoprim and sulfamethoxazole through 2011. Considering that the vast number of medication adverse reactions are rarely identified much less published, almost 1000 papers published suggests something is going on with this medication. While most of the adverse effects of this drug are attributed to its induction of hyperkalemia – high potassium levels via its blockage of the sodium channels in the kidneys, I think that is only part of the story. Another component, as I mentioned previously, involves its ability to block critical nutrients; first as an anti-folate, per its original design, and next quite incidentally, at least as far the published research is concerned, as a thiamine transporter antagonist. The blockade of the metabolism and uptake of these two critical nutrients is key to understanding the spectrum of adverse events associated with it, particularly those not directly induced by the sodium channel blockade that causes hyperkalemia. Although I would argue, deficiencies in these two nutrients would exacerbate Bactrim’s effects on sodium-potassium balance.
Bactrim Blocks Bacterial Folate Metabolism
Folate, or vitamin B9 is essential for DNA synthesis and repair. As such, it is critically important during reproduction for all organisms from bacteria to human. Folate deficiency during pregnancy is associated with serious neurodevelopment aberrations including neural tube defects like spina bifida and thus Bactrim should be strongly contraindicated for pregnant women, but at least one small study found that 3.2% of the pregnant women sampled were prescribed Bactrim.
Folate deficiency at any point across the lifespan may be equally problematic, though less frequently observed, as it provokes an array of symptoms that may attributable to any number of factors. Nevertheless, folate deficiency is linked to immune system dysfunction, dermatological issues, cardiovascular dysregulation, and neurologic problems, including sensory neuropathies with axonal damage. This is addition to a spectrum of neuropsychiatric manifestations from depression to dementia that are associated with this critical nutrient. Importantly, folate and folate products are linked to almost every other metabolic pathway through shared ATP, NAD, and NADP pools inasmuch as they both contribute to the production of NADPH and ATP, and are directly affected by the ratios of these molecules. Folate and vitamin B12 share a particularly close relationship, where deficiency in one, creates a functional deficiency in the other via alterations in methionine pathway leading to megaloblastic anemia. All told, folate is integral for healthy cell function and proliferation, mitochondrial respiration and epigenetic regulation.
Each of the two drugs that make up Bactrim block microbial folate synthesis precipitating complete folate deprivation in bacteria, capable of resulting in folate deficiency non-bacterial cells.
The trimethoprim component of Bactrim binds to a critical enzyme in the metabolism of folate, called dihydrofolate reductase (DFT). This inhibits the reduction of folate into cofactors necessary for DNA synthesis. By binding DHT, dihydrofolic acid (DHF) and then tetrahydrofolic acid (THF) are blocked. THF is essential in one carbon metabolism and the transfer of methyl, methylene, and formyl groups from one molecule to another during the production of nucleotides and amino acids e.g. DNA synthesis and repair.
Sulfamethoxazole also blocks folate metabolism, albeit at a different junction. It is a structural analog of the vitamin-like compound para-aminobenzoic acid (PABA) found in several foods and involved in the metabolism of folic acid. In bacteria, it is a required growth factor. As a structural analog to PABA, sulfamethoxazole binds to and blocks a key enzyme in the folate pathway (dihydropteroate synthetase) thereby inhibiting the conversion of PABA and downstream metabolites critical for folate synthesis and metabolism.
Neither trimethoprim nor sulfamethoxazole alone kill bacteria. They simply prevent bacterial replication. Taken together, however, the combination yields potent bactericidal effects. Bactericidal antibiotics, as a class of drugs and irrespective of specific mechanisms of action, fundamentally and sometimes irrevocably, damage mitochondria.
…it has been demonstrated that major classes of bactericidal antibiotics, irrespective of their drug-target interactions, induce a common oxidative damage cellular death pathway in bacteria, leading to the production of lethal reactive oxygen species (ROS) (4–12) via disruption of the tricarboxylic acid (TCA) cycle and electron transport chain
The overall importance of these observations relates to an expanded mechanism of action, whereby bactericidal antibiotics promote complex redox alterations that contribute to cellular damage and death, while also underlining a common evolutionary and developmental linkage between primordial bacteria and mitochondria (56,57).
Damaged mitochondria, in turn, imperil human health and no doubt, contribute to the vast array of post-antibiotic health issues that have become increasingly common and associated with a number of antibiotics.
Bactrim Blocks Thiamine Uptake
In addition to blocking folate metabolism, the trimethoprim component of Bactrim also blocks thiamine uptake. This was only recently discovered by accident. As part of a study on a cancer drug called Fedratinib, which is known to induce a severe form of thiamine deficiency called Wernicke’s encephalopathy, researchers tested the thiamine blocking capabilities of several drugs that were structurally analogous to Fedratinib. Trimethoprim was among the drugs tested and found to potently block both thiamine transporters. Absent the ability to transport thiamine from diet into the cells, deficiency ensues.
Like folate, thiamine or vitamin B1, is an essential cofactor for key enzymes involved in one carbon metabolism and energy production in all living cells. Thiamine acts as a catalyst and cofactor to all of the enzymatic reactions that participate in oxidative metabolism yielding ATP (see figure 1) and is absolutely critical for glucose metabolism. It also occurs twice in the pentose phosphate pathway (PPP), the alternative glucose oxidation pathway that provides nicotinamide adenine dinucleotide phosphate (NADPH) and ribose 5-phosphate (R5P) for glutathione, nucleic acid, and fatty acid synthesis and steroid hydroxylation, respectively, making thiamine necessary for not only ATP production, but required for duplication and detoxification processes. It is also involved at the alpha oxidation phase of fatty acids, at the HACL1 enzyme and is critical for the metabolism of the branched chain amino acids, leucine, isoleucine, and valine.
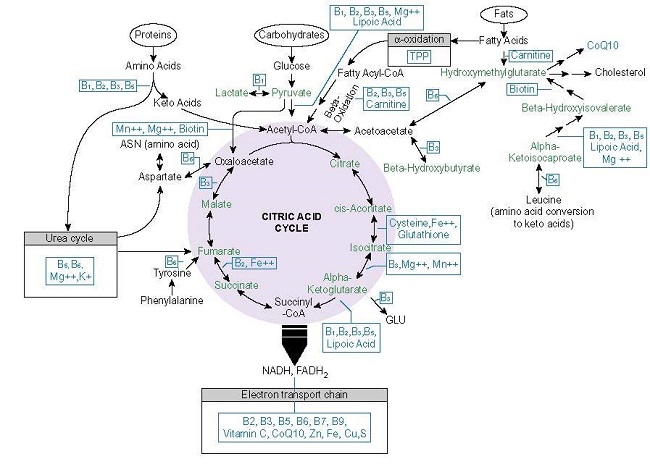
Importantly, thiamine sits atop these processes, as a gatekeeper of sorts. Its absence or insufficiency thus, derails downstream functions associated the conversion of food into ATP in each of the substrate pathways, glucose, protein, and fats, creating a biological energy deficiency that imperils human health. Insofar as thiamine is only stored in the body for about 18 days, it must be consumed regularly to avoid insufficiency and outright deficiency. Insufficient consumption and/or ingestion of pharmaceutical compounds that block intestinal thiamine transporters responsible for bringing thiamine into the cells, pose a serious health risk that includes death. Mitochondrial ATP is requisite for cell functioning globally, as such decrements to ATP affect every organ and tissue in the body, but are most damaging where a consistent supply of ATP is requisite for survival – in the heart and the brain. That is why, the most serious conditions associated with thiamine deficiency are wet and dry beriberi and Wernicke’s encephalopathy, the condition for which Fedratinib contains a black box warning. More commonly, thiamine insufficiency is associated with a litany of dysautonomic syndromes, although it is not widely recognized as such.
The case story that compelled my investigation into Bactrim included clear symptoms of both wet and dry beriberi, marked by serious dysautonomic function. The bradycardia, chaotic heart rhythm and blood pressure changes were the most acutely dangerous for this individual, particularly if this was accompanied by disrupted sodium and potassium balance, as we can suspect was the case. Had he been older and/or carried additional comorbidities, he might not have survived. He did survive, however, but remains chronically symptomatic of thiamine deficiency 6 years later.
The question one must ask is how presumably healthy individuals develop thiamine insufficiency upon the usage of drugs like Bactrim. That is, how are Bactrim and other thiamine depleting drugs capable of provoking such a rapid decline into fulminant deficiency? Thiamine is, after all, in most enriched and fortified foods. A similar question, how then, even after cessation of the drug do these individuals develop unremitting health issues indicative of longstanding thiamine deficiency?
Absent outright thiamine starvation, most folks consume sufficient thiamine from food to avoid the more acute thiamine deficiencies, but not enough to prevent the more gradual and often chronic thiamine insufficiency syndromes that mark modern medicine and certainly not enough to offset the direct blockage of thiamine transporters from pharmaceuticals like Bactrim, the mitochondrial damage initiated by modern medications (here, here, here, here, and more), environmental chemical exposures and industrial food based toxicants to which we are all exposed. If one’s diet is high in sugars, supplemented with coffee or tea, and/or if alcohol is consumed regularly, the path to thiamine deficiency is expedited.
From this perspective, it is not inconceivable that a significant portion of the population is thiamine insufficient, if not outright deficient. Across different research projects, estimates suggest from 30-70% may have insufficient thiamine intake to meet the demands of daily living and given the corresponding rise in chronic health issues of metabolic e.g. mitochondrial origins, it is not surprising that many are just one medication away from full blown deficiency. To the extent that thiamine is connected to the synthesis of downstream metabolites like folate synthesis, it is not unexpected either, that a background insufficiency in both nutrients would be exacerbated and potentially become deadly with the addition of Bactrim to the mix. Moreover, absent nutrient repletion post antibiotic usage, it is entirely likely that the mitochondrial ill-effects imposed by this drug would become longstanding.
Of Bacteria and Men: A False Dichotomy
Although trimethoprim’s thiamine blocking capability was not known until 2017, had anyone bothered to look at the structure of the compound relative to that of thiamine, it would have been obvious. As far as I can tell, in the 50 years this drug has been on the market, its conformational similitude to thiamine was not considered. Nevertheless, it was well known that Bactrim blocked folate, that folate was critical to human health, and that its deficiency could wreak havoc on health. Why was its actions on folate metabolism not considered problematic? The answer to that question has to do in part to shoddy research and in part to an economically self-serving framework for understanding human physiology that has since become institutionalized into medical dogma. In other words, it was easier and more economically prudent not to question potential problems in the research or the assumptions driving said research than risk losing a useful and lucrative antibiotic.
When trimethoprim was originally discovered and yet still, medicine believed that bacteria were somehow entirely separate from the organism in which they resided. The otherness of potential drug targets holds true to this day.
Although trimethoprim inhibits dihydrofolate reductase in bacteria, it is estimated that an approximately 50,000 times increased concentration of the drug is required to inhibit the human form of this enzyme.”
This notion appears to be based upon a study in 1965, where uptake of the drug and subsequent DHT enzyme binding activity in lab grown bacteria (escherichia coli, staphylococcus aureus, proteus vulgaris), in other animal tissue, and in pulverized human liver cells post autopsy from one individual, were compared. The bacteria won. When trimethoprim was present, folate to DFT was not reduced, even when extra folic acid was added to the media. Remember, in order to get to usable folate – THF, we need the DFT enzyme to work. So, based upon the blockage of the DFT>THF pathway in bacteria, trimethoprim was deemed ‘strongly antibacterial’. Furthermore, it was deemed safe by its apparent inability to block folate in ‘human cells’. It should be noted that the stability of the enzymes from the animal and the human liver cells varied significantly from 25-60%, calling into question the very results upon which this medication was eventually developed and approved. No matter, a potential antibiotic was born and no one was the wiser.
The fallacies upon which trimethoprim safety was based were that bacteria are completely separate from the humans in and on which they reside, that they are solely responsible for illness, and that pharmaceuticals designed to attack said bacteria would affect only their intended targets. None of which are true. While it is true that some of the enzymes within the metabolic pathways in bacteria are different from those in other cells, they still share a degree of similarity, some 30% sequence homology, suggesting enzymes in these cells will also be affected, though perhaps not as strongly as those in the bacterial populations.
More importantly, however, and this speaks to the fallacy of separateness that medicine holds dear, at any given time, we carry with 3–6 pounds of commensal bacteria that are responsible for a myriad of functions, including “protective responses that prevent colonization and invasion by pathogens,” the inhibition of “growth of respiratory pathogens by producing antimicrobial products/signals and competing for nutrients and adhesion sites” and importantly, for our purposes, the synthesis and metabolism of vitamins to be used by the host; the very pathways blocked by these antibiotics.
Several intestinal bacteria in the colon, but also in the small intestine, are capable of biosynthesis of natural forms of folate as well as vitamin B12 and other B-vitamins (Camilo et al. 1996; Magnusdottir et al. 2015; Rossi et al. 2011). It has been shown that specific transporters in the colon actively absorb folate (Said 2013) and as such contribute to folate levels in peripheral tissues and the circulation (Aufreiter et al. 2009; Lakoff et al. 2014; Pompei et al. 2007b). These findings indicate that intestinal bacteria contribute to folate metabolism and that colonic contents represent a substantial and natural source of folate.
We are neither separate from our bacterial communities nor are our vitamin synthesis pathways sufficiently distinct from bacteria that we can target a pathway in one without affecting the other. We carry vast microbial ecosystems whose functions are critical to human survival; vitamin synthesis among them. Indeed, bacterial folate synthesis genes are ubiquitous across the gastrointestinal tract, 13% of which, contain all of the required for complete de novo folate synthesis and almost 40% have the genetic capacity to synthesize folates in the presence of PABA, the upstream intermediate blocked by the sulfamethoxazole component of Bactrim. Importantly, bacterial synthesis of folate and other B vitamins, represents a critical pathway not only for nutrient availability of the human host, but for managing the vast microbial ecosystems in a manner favorable to host survival. Disruption of these ecosystems involving nutrient depletion results in pathogenesis both of the infectious and oncogenic varieties.
Given the large number of gut bacteria in comparison to eukaryotic cells, which also contain evolutionary derived mitochondria (mitochondria are believed to originate from bacteria and as such share similar enzymes and metabolic pathways with them), it would seem that the blockade of these critical nutrient pathways while effectively antibacterial, may also, induce unintended harm. Materially,
…the summated populations of ‘simple’ organisms may in fact regulate the ultimate fate of our genetic material. In sum, it has become compellingly apparent that eukaryotic cells and complex organ systems cannot survive without the synergistic complex interactions of competent enteric bacteria and evolutionarily fashioned mitochondria…
but “go ahead and just cover him with some Bactrim. How can it hurt?”
Postscript
Bactrim is also sold under the names: Septra, Sulfatrim, Septrin, Apo-Sulfatrim, SMZ-TMP and cotrimoxazole.
Although I did not spend any time covering Keflex, the second drug prescribed to this individual, I would like to note that it too damages mitochondria, albeit by different mechanisms. Briefly, Keflex can cause a deficiency in leucine, an amino acid that regulates something called the mTOR receptor, which, when blocked or, in this case, absent its cognate ligand, would downregulate mTOR and deregulate mitochondrial function. The mTOR pathway regulates the balance between protein anabolism and catabolism critical for cell growth and division. Its down-regulation shifts towards catabolism resulting in muscle wasting.
If you experienced a negative reaction to Bactrim and would like to share your story, contact us.
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
Photo by Haley Lawrence on Unsplash.
This article was published originally on October 20, 2020.












Praise to the Lord I found this article! I suffer from impaired thiamine metabolism but am currently on my third day of treatment with bactrim and keflex for a nasty cellulitis infection in the ball of my foot. The drugs are causing dyspnea, unusual fatigue, severe insomnia and other things too numerous to list. I suspected this was the case and your article confirms it. Please advise asap on what alternative antibiotics, if any, or mix of supplements can help me to get past this? I do have allithiamine, magnesium and active forms of b vitamins as a multi b vitamin complex, as well as colloidal silver and an antibiotic product called Allicin. Thanks in advance for any guidance you can provide.
I have said for years keflex messed me up. I was right. This is a missing piece of my health journey! Thanks for this article.
Hi, I was wondering how you feel Keflex has messed you up?
Thanks
Thank you so much for this article. I just finished a five day course of 800mg bactrim DS for a uti. I had the rapid heart rate, insomnia , indigestion, and the worst still after finishing is my red gums, red and sore tongue. I can hardly eat ! I am running out to pick up some vitamins asap. I do not understand why this is not more well known. I am just so wary of medications these days. I will be more in tune and reading your blog for sure.
Dear Doctor, I would like to express the deepest gratitude to you for writing and updating this website. You are doing an amazing job and helping lot’s of people with it! I’m a student from another country and your blog is a real treasure to me, as it helps me to understand pathological processes and their connection with biochemistry and other academic disciplines better. Thank you very much for your job ❤️
Hello,thank you for the information.40 years ago just one Bactrim put me in hospital.All over mucuos membranes were red,open sores and painful.I was student in the university,went to hospital,internal med.dr saw me and called for a consultation from other departmans,they realize it was Bactrim allergy and said I should be in the literature.For one week I could only take water with pipet, going to bathroom was really really painfully was crying.
Because of my allergies I took a Penicillin test and I reacted.
Years later I was giving Cipro.With Cipro I had Ulnar nerve entrapment and muscle atrophy.
Thank you so much for this information. After just a few days on Bactrim I started experiencing rapid heart rate, hyperventilation-type symptoms, numbness and insomnia. I recognized it immediately as a need for thiamine. I’m typically somewhat deficient already due to hyperthyroidism and wondered why the sudden increase in symptoms. Thank you for writing this. It confirms what I had suspected that it was the Bactrim. I’m lying awake as we speak due to these symptoms and stumbled across your article trying to figure out what was wrong. I’m stopping this medication ASAP and taking a full dose of thiamine today.
You’re welcome.