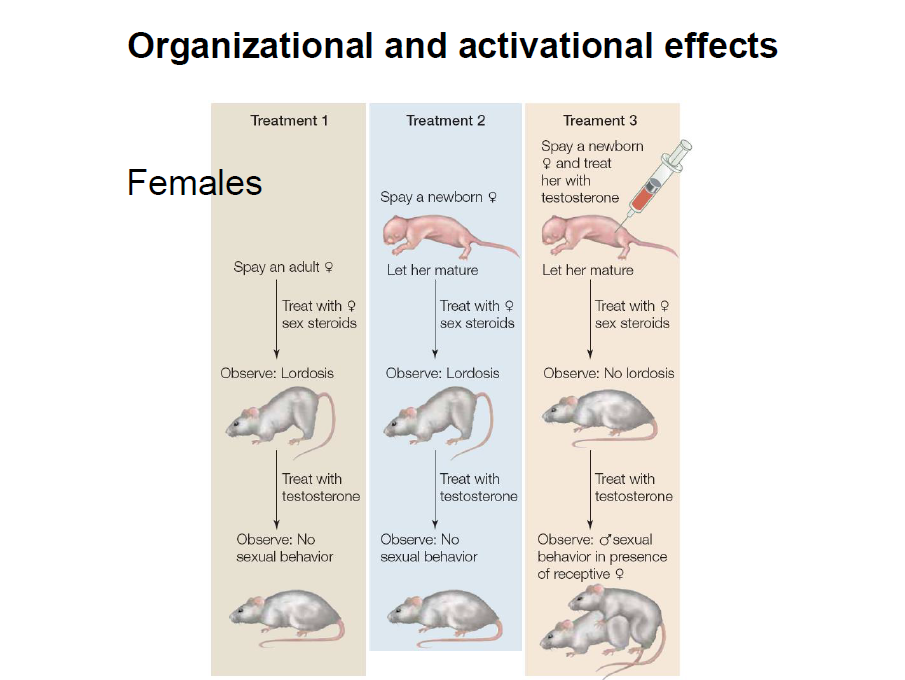
“The doctor wouldn’t have given it to me if he thought it was dangerous, right?”
My wife asked this salient question as we discussed the pros and cons of The Pill. It sent us both into deep reflection. Everything we read said The Pill was dangerous, but the doctor had acted like they should come in a Pez dispenser. To this day, I’m not sure where the cognitive began and the dissonance ended.
The DES Debacle: Origins of Obstinance
Doctors are generally dogmatic, but their nearly universal laissez-faire attitude toward The Pill seems particularly paradoxical when you study the scope and seriousness of its side effects. How can doctors believe that The Pill is safe, when tomes of studies suggest otherwise? Research links The Pill to everything from breast cancer and strokes, to Crohn’s Disease and lupus. To understand where we are and how we got here, it’s important to study the journey that brought us here.
By 1970, the current dogma that ‘The Pill is safe’ was well rooted in the medical community. However, enough doctors expressed concerns that Senator Gaylord Nelson decided to hold Congressional Hearings on the matter. The big three networks covered the hearings extensively, which caused great anxiety among women taking The Pill — and even greater anxiety among pill proponents, who subsequently demanded more ‘pro-pill’ doctors be included.
Senator Nelson took umbrage with their complaints, noting that all but one of the previous doctors had actually been ‘pro-pill’ to some extent, but all had reservations about its complications. Nonetheless, many of the doctors in the second round of hearings seemed more decidedly ‘pro-pill,’ including Dr. Kenneth Ryan, who stated,
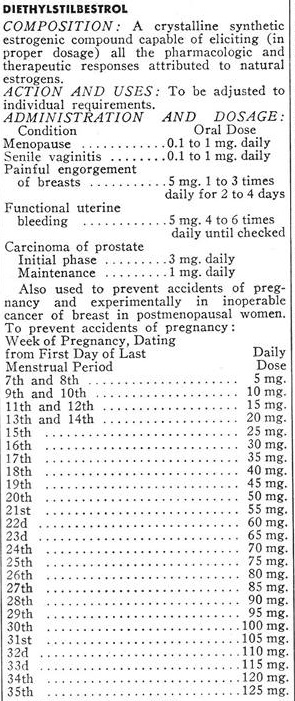
I know of no information that indicates that biological properties of the estrogens used in the contraceptive pill are any different than stilbesterol for which we have at least 30 years of clinical experience…(Competitive Problems in the Drug Industry, Ninety-First Congress, Second Session, Page 6541)
Very reassuring… Unless you were actually familiar with the 30-year history of stilbesterol, also known as diethylstilbestrol (DES). Sir Charles Dodds discovered DES in 1938, and rushed it to market in the public domain. The English doctor bypassed the patent process hoping it would discourage the Nazis from further tests on women prisoners in their development of ethinyl estradiol (Barbara Seaman, The Greatest Experiment Ever Performed on Women; page 36).

Despite his noble intentions, Dodds soon regretted the decision. Without a patent, drug companies around the globe were free to produce DES. He never expected that synthetic hormones would be given to healthy women, and was horrified that doctors were prescribing it as hormone therapy for natural menopause.
You Can’t Put the Hormones Back in the Tube
Even worse, Dodds soon learned that an American doctor named Karnaky had begun blazing a new trail – doling out DES to ‘prevent miscarriages’. Alarmed by the news, Dodds sent him a study he had personally performed, which showed that the drug actually caused miscarriages in animal subjects. It didn’t deter Dr. Karnaky or the many doctors who followed his lead. (Robert Meyers, D.E.S. The Bitter Pill; pp. 56-73)
Dodds began to feel like he was fighting a monster that he himself had unleashed. He was most concerned about how his discovery could affect certain cancers. He sent DES samples to the newly formed National Cancer Institute in the United States, and urged them to conduct tests and notify doctors.
Dodds wasn’t alone. The Council on Pharmacy and Chemistry warned,
…because the product is so potent and because the possibility of harm must be recognized, the Council is of the opinion that it should not be recognized for general use at the present time…and that its use by the general medical profession should not be undertaken until further studies have led to a better understanding of the functions of the drug. (Barbara Seaman, The Greatest Experiment Ever Performed on Women; page 44)
The concerns sent murmurs through the medical community, and many doctors began experimenting with lower doses of DES. By 1940, the editors of the Journal of the American Medical Association (JAMA) felt compelled to add theirs to the litany of warnings:
“It would be unwise to consider that there is safety in using small doses of estrogens, since it is quite possible that the same harm may be obtained through the use of small doses of estrogen if they are maintained over a long period.” (JAMA, April 20, 1940)
In 1959, the FDA determined the link to side effects (including male breast growth) was sufficient to ban poultry farmers from using DES as a growth hormone. However, the widespread use of DES in humans continued. In fact, it had become standard medical practice by the time Dr. Ryan assured Congress that The Pill was just as safe as DES – showing how medical dogma often trumps scientific evidence.
The greater irony of Dr. Ryan’s statement materialized one year after his testimony, when researchers first linked a rare vaginal cancer to the daughters of women who received DES during pregnancy. The FDA reacted strongly, listing pregnancy as a contraindication for DES use.
Consumer Groups Take the Lead
You would expect this to be the beginning of the end for DES. Shockingly, the medical community responded with indifference, continuing to prescribe DES for a variety of ‘off label’ uses, including as a morning-after pill, to catalyze the onset of puberty, and the old faithful, hormone replacement therapy. (Robert Meyers, D.E.S. The Bitter Pill; page 185)
It took nearly a decade of passionate effort from consumer movements like DES Action to convince doctors to (mostly) abandon DES. Dozens of lawsuits were filed; some were settled; and some are still pending. There is evidence that the harmful consequences could now be affecting a third generation of DES victims.
The current Director of Epidemiology and Biostatistics at the National Cancer Institute, Robert Hoover, M.D. oversees the DES Follow-Up Study to track the ongoing repercussions. With identifiable problems now affecting the grandchildren of women who took DES, the disaster hasn’t yet moved into the past tense of our nation’s history. Despite that, Dr. Hoover says:
There’s essentially a whole generation of medical students who don’t know the story. The story has such powerful lessons that I think that’s a tragedy…For about 20 years now, when I standardly ask in my general epidemiology lecture… how many of you have heard of DES, nobody raises their hand.
Sidney Wolfe, M.D., who headed up Ralph Nader’s Health Research Group offered this perspective,
DES is an excellent example of how drug companies behave, how they take advantage of the ways doctors act, and how they make millions of dollars by ignoring evidence of a drug’s harmfulness, by failing to get evidence that it is effective, and then by marketing a product that plays on fears and misconception. (Robert Meyers, D.E.S. The Bitter Pill; page 208).
In just 20 years, the American Medical Association moved from “It would be unwise to consider that there is safety in using small doses of estrogens…” to embracing the release of insufficiently tested hormones as birth control for millions of women. I’m leery of trusting a dogma founded on such an erratically moving target. In their defense, the dogma really hasn’t moved much in the decades since.
Today, the medical community assures us The Pill is the most researched drug ever. Sorry doc, that reassurance just doesn’t ring true. At this point, it feels more like a phrase learned by rote than a statement based on any kind of empirical evidence. Unfortunately, it’s not the only hollow mantra that should raise a red flag when it comes to hormonal contraceptives. I will discuss how the medical community responds to scientific studies in my next post, The Spin Doctor’s Prescription for Birth Control.
[atkp_product id=’48881′ template=’bestseller’][/atkp_product]
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
This article was published originally on Hormones Matter on August 31, 2016.













 DES is not something of the past. People who have been exposed to this drug years ago are battling with health issues and fighting for their lives as I’m writing this blog post. Who knows what health problems the grandchildren of the mothers who were prescribed this drug will have to deal with as they grow up. I want my daughters to receive adequate medical care and monitoring if they ever have to suffer the consequences of this drug. This is why together with my husband we support the great work done by the very few International DES Action Groups who are providing valuable information and are advocating for the DES victims.
DES is not something of the past. People who have been exposed to this drug years ago are battling with health issues and fighting for their lives as I’m writing this blog post. Who knows what health problems the grandchildren of the mothers who were prescribed this drug will have to deal with as they grow up. I want my daughters to receive adequate medical care and monitoring if they ever have to suffer the consequences of this drug. This is why together with my husband we support the great work done by the very few International DES Action Groups who are providing valuable information and are advocating for the DES victims.