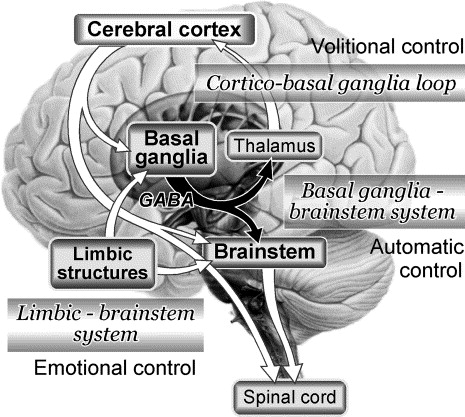
I am 41 years old and experiencing weird, scary, and upsetting symptoms that began a year and half ago. I have numbness in my feet that has moved up into my calf and super tight calves, ankles and feet. I have peripheral neuropathy, carpal tunnel symptoms, circulation issues without swelling and some sciatica like symptoms with achiness in my legs. I am exhausted all of the time, have no energy and symptoms of depression, but I am not depressed other than these health issues. I am overly irritable and always cold. My hair is falling out and I become dizzy when I standup from a sitting position.
In the past, I believe at some point I had insulin resistance but was never diagnosed. I did, however, have issues with my blood sugar. I was put on Metformin for 6 years, during which time I had a lot of stomach issues, nausea, and diarrhea. I was never a big drinker at all, nor did I use tobacco. I was under a ton of stress for many years though. Despite the stress, I was always healthy and worked out, ran on a treadmill, and was active. I worked as a nanny 55 hours a week.
My diet before metformin was not the greatest with lots of carbs and processed foods. I may have had a thiamine deficiency back then and but did not know about it. No one ever tested me for thiamine until recently. A lot of my symptoms started at a time where I was dealing with some heavy things, so I believe stress was definitely involved. For the past three years, I have not been on medication. Currently, I eat a low carb/keto diet and my A1c is 5.2 and insulin is 3.
Discovering Thiamine Deficiency
I started to experience these symptoms about a year and a half ago. I have tried many things to feel better and help with my symptoms and nothing has worked. Of the nutrient testing that I have had, my thiamine was low. It was 66nmol/L. The reference range was 78-185nmol/L. My vitamin D was barely above the deficiency range at 30ng/mL, my methylmalonic acid was on the low end of the range at 107nmol/L (range 87-318), and my vitamin B6 was high at 29.5ng/mL (range 2.1-21.7). Nothing was discussed regarding the other low vitamins and high B6. I was, however, told by my neurologist to take 100mg a day of vitamin B1/thiamine. She never indicated that this was the reason for my symptoms though.
I began doing my own research and found that I had all of the classic symptoms of dry beriberi – thiamine deficiency that affects the nerves. In other words, my symptoms were related to thiamine deficiency. I began supplementing with Benfotiamine 600mg a day am taking magnesium (Optimum health) at 150×2= 300 at night. My FM doctor said my magnesium was at 4.5 and they like to see it at 5.3. I also take vitamin D3/K2. My vitamin d was on the low side.
When I began supplementing with thiamine at 100mg per day and the Benfotiamine, I notice I was not as tired or fatigued. I was feeling pretty weak there, and I feel better, but the nerve issues have not changed.
Six weeks after finding out I had a thiamine deficiency, I got bloodwork from my FM doctor and my thiamine was now too high, almost as if I wasn’t absorbing it. I should mention that the second test was a plasma test while the first test was done from the serum. From what I have learned, plasma thiamine measures are less accurate. Even so, should I be worried?
My FM doctor wants to test again for Lyme disease. Beyond that, I just don’t know what else to do to resolve the nerve issues. Thank you!
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
Image by Daniel Reche from Pixabay.
This story was published originally on October 5, 2023.




























 Indeed, there have been many relapses over the years, where her health declines significantly and requires extended periods of recovery. Nevertheless, she is able to recover and that is remarkable.
Indeed, there have been many relapses over the years, where her health declines significantly and requires extended periods of recovery. Nevertheless, she is able to recover and that is remarkable.