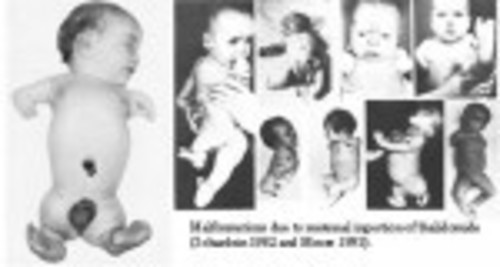
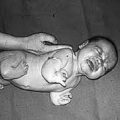
Fifty years ago, if you were pregnant you might have been prescribed or bought thalidomide over the counter for morning sickness. While the drug was labeled “harmless,” if a woman took this drug while pregnant, there was a good chance that she would have a child with phocomelia, or seal limbs. One of the many dangerous side effects was a birth defect where the bones of the arms and legs fail to develop properly and literally look like seal flippers.
Origins and Side Effects
Thalidomide was developed in Germany in 1957 and was considered safe enough to be sold over the counter in some countries. It was sold in 45 countries. The FDA in the US never approved it, but there were victims who were part of a clinical trial where 20,000 patients received the drug, as well as patients who obtained the drug abroad.
In 1967, the drug was pulled from the market. It is now reported that 10,000 – 20,000 babies were born with severe birth defects during the 5 years that it was on the market.
Martin W. Johnson, director of the Thalidomide Trust of Great Britain, stated that about 40 percent of babies with thalidomide-induced defects died before their first birthday, and approximately 50 percent of those living today live with chronic pain. Of course, phocomelia isn’t the only birth defect; many other babies suffered heart problems, damaged hearing or eyesight, and even brain damage.
Apology – Too Little Too Late?
It’s been over fifty years since this tragic drug mishap and the CEO of Gruenenthal, Harald Stock, is finally apologizing to the victims. Is it enough?
Geoff Adams-Spink, born in 1962 with multiple impairments caused by thalidomide and a BBC journalist for 22 years, wrote an Op-Ed piece about this apology stating:
“The Wirtz family [predecessors to Harald Stock] has grown fat on the backs of thousands of families whose lives have been torn apart by a medicine originally marketed as “totally without harm.” If they really want to make amends, they should put their entire wealth at the disposal of the world’s thalidomide survivors before it’s too late.”
He’s not alone; many of the victims feel more insulted than compensated by this financially absent, PR stunt of an apology. This includes an Australian woman, Lynette Rowe, who recently won a multi-million dollar settlement in July against Diageo Plc, the legal successor to thalidomide’s Australian distributor. Wendy Rowe, Lynette’s mother who took the drug while pregnant, told journalists, “Our family couldn’t have gone into silent shock. We had to get up and face each day and every day and cope with the incredible damage that Gruenenthal drug did to Lyn and our family.”
Today’s Uses and the Future of Thalidomide
In 1964, Israeli scientist, Jacob Sheskin, discovered thalidomide could control leprosy by reducing the inflammation caused by the disease. In 1998, the FDA approved it for multiple myeloma, a cancer of plasma cells in the blood. Because leprosy is still a serious problem for populations in Africa and South America, there are women taking thalidomide as treatment and giving birth to babies with phocomelia and other birth defects. Due to this ongoing use and therefore side effects, scientists have continued to study why thalidomide is so dangerous. They recently discovered that the protein cereblon latches on to the thalidomide and is a major reason for the tissue damage in the fetus.
In the US, thalidomide is used, but under extreme restrictions for both men and women. The FDA has put in place the System for Thalidomide Education and Prescribing Safety (S.T.E.P.S.®) to make sure that pregnant women do not take thalidomide and that women do not become pregnant while taking thalidomide. According to PubMed Health: “All people who are prescribed thalidomide, including men and women who cannot become pregnant, must be registered with S.T.E.P.S.®, have a thalidomide prescription from a doctor who is registered with S.T.E.P.S.®, and have the prescription filled at a pharmacy that is registered with S.T.E.P.S.® in order to receive this medication.” Furthermore, it is only prescribed one month at a time and a doctor’s visit is required for additional prescription. Women must use two acceptable forms of birth control for four weeks prior to taking the drug. A weekly pregnancy test is also required every week for the first month and monthly thereafter if you have regular menstrual cycles (every two weeks if you have irregular cycles). And for the men, you are instructed to use a condom because thalidomide can be transferred through the sperm causing birth defects as well.
What about Other Victims of Other Drugs
Fifty years and a verbal apology is all the victims of thalidomide have received. The apology stated:
“We also ask for forgiveness for not reaching out to you from human to human for almost 50 years … We ask that you see our long speechlessness as a sign of the silent shock that your fate has caused us.”
This is what happens when we do not hold the pharmaceutical or medical device industries responsible for their products. It makes me wonder how long it will take before Gardasil and Cervarix are taken off the market? How long will it take for the victims, like Alexis in A Life Ruined by Gardasil, who suffer from mild to severe side effects, including death, to receive any sort of compensation, if ever? What about other drugs with more side-effects? To the victims of thalidomide the apology might not seem like enough, but I see it as an admittance of guilt that the company, and those who approved the drug, put profits above safety. This apology is a start and maybe someday soon we’ll see more pharmaceutical companies taking responsibility for the damages they leave on their patients.
The photo is a work of the National Institutes of Health, part of the United States Department of Health and Human Services. As a work of the U.S. federal government, the image is in the public domain.