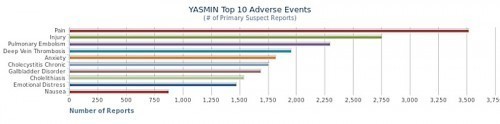
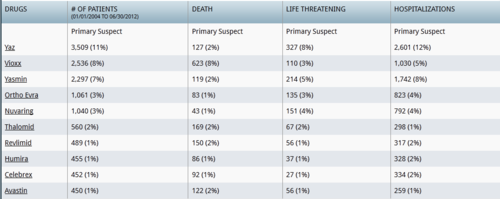
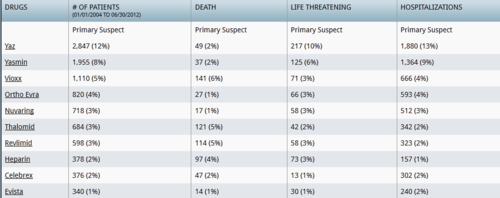
As a firm believer in natural and holistic health, I believe that the food you eat can have a direct impact on your health. For those dealing with reproductive and or endocrine disorders simple dietary changes may help prevent or relieve a number of troublesome symptoms. For women across the board, especially those with estrogen sensitive diseases (e.g. endometriosis) minimizing soy in one’s diet can provide a lot of relief.
But, isn’t soy good for you? That’s not an easy question to answer. In moderation soy can provide a lot of benefits to one’s diet. However, soy should not be a staple in your day to day meal planning. To better answer the question we need to break it down.
Phytoestrogens
Soy is filled with phytoestrogens also known as “dietary estrogens,” which mimic the hormone estrogen that is naturally produced by the human body. Research has shown that phytoestrogens are beneficial for women going through menopause, since menopause is marked by a reduction in estrogen levels. Some menopausal women say that eating soy products helps relieve some symptoms of menopause such as hot flashes. Research has shown that phytoestrogens help maintain cholesterol levels and bone density in post-menopausal women. In addition phytoestrogens also help protect against a number of different cancers, such as prostate and breast cancer, cardiovascular disease and help with cognitive functioning.
However, soy being filled with phytoestrogens, can also be bad for you. Estrogens are female sex hormones – estradiol is the most common. Estradiol promotes the growth of female sex characteristics. Soy is also high in phytic acid which if consumed in high quantities can affect the absorption of other minerals. That is why third world countries that survive on grain and legume based diets have high rates of mineral deficiencies.
Too Much Dietary Estrogen
Increased estrogen consumption can be particularly detrimental to a number of people (i.e. babies, children, men, women sensitive to estrogens). Studies have shown that giving babies/infants soy formula is the estrogenic equivalent to giving them daily birth control pills. Obviously, such small children shouldn’t be on any form of contraception. It has also been suggested that this increased ingestion of estrogen can lead to estrogen sensitivities, earlier onset of puberty and reproductive diseases/disorders such as endometriosis. For older children increased amounts of estrogen also cause pubertal issues. Large quantities of estrogen can delay puberty for boys and lower the age of menarche (first menstruation) for girls. The average age of menarche decreases every year and a lot of researchers and medical professionals hypothesize that is has to do with the environment, toxins and the estrogen that comes from outside sources. Early puberty can also lead to short stature in women.
Estrogen in abundance is also bad for grown men. Men produce both testosterone and estrogen, with testosterone being the predominant hormone by far, and responsible for masculine features. Too much estrogen in males can offset their testosterone to estrogen balance, causing hormonal deficiencies. Lastly, estrogen is bad for women who are sensitive to estrogen, such as women with endometriosis. The endometrium that builds up in endometriosis responds to estrogen. The more estrogen you give your body the more the endometrium can build up, which leads to additional inflammation and bleeding.
Not all Soy Products are Created Equal
If you are still confused as to how soy can be both ‘good’ and ‘bad’ for you consider this: soy products come from a soy bean – however, if you take a good look at your soy-patties or chips made with soy you won’t see mashed up green beans. The invisible soy you eat come from processing the beans. ‘Asian’ soy which is usually quoted as being “good” soy is fermented soy. The process of fermenting soy can reduce a lot of the detrimental qualities of soy, but not entirely. Unfermented soy contains antinutrients such as tripsin inhibitors which can produce serious gastric distress, reduced protein digestion and create chronic deficiencies in amino acid uptake. When soy is processed and manufactured it isn’t always fermented. There are two popular forms of processed soy:
Soy Lechitin: Soy lecithin is basically a waste product of refining soybean oil. In small amounts it isn’t harmful, but if you look at food labels it is found in a large amount of products. Putting soy products in everyday foods provides a lot of nutritional benefits (lower fat, lower calories, higher protein) while being cost effective to the manufacturers. Unfortunately when you process soy it also increases the levels of estrogens and goitrogenics. Goitrogenics decrease the productivity of the thyroid and are bad for those with thyroid disease. Although ironically enough lecithin by itself (not soy lechitin just plain old lechitin) is good for you, it is a natural product high in choline.
Soy Protein Isolate: Soy Protein Isolate is the key ingredient in most imitation meat and dairy products. It is also found in other manufactured goods and protein powders. To make soy protein isolate, the soy beans must first be mixed with an alkaline solution to remove fiber, then rinsed and separated using an acid wash and, lastly, neutralized in an alkaline solution. The resulting products are finally dried at high temperatures to produce a high-protein powder. A similar process is used to make textured vegetable protein which is a high-temperature, high-pressure extrusion processing of soy protein isolate. With natural almost always being better for you, a process like this is by no means healthy. It doesn’t sound good for you and it isn’t. This process can also increase the amount of trypsin inhibitors.
So… Is soy good for you? Studies show that a glass of red wine at dinner is good for you. But we all know that washing dinner down with a bottle of red wine every night would not be good for us. Approach soy in the same way; some soy products several times per week, especially if it is fermented, organic soy (think tofu cubes and not ‘fake pepperoni’) will provide more benefits than detriments. In general be smart about what you put in your body, be an educated consumer and read your labels. Just because it has soy doesn’t make it good. But, that doesn’t mean you need to take all soy products out of your life.