The Role of Copper in Mitochondrial Function
Copper is an essential trace element required for the propagation of optimal enzymatic activity and electron transfer in the cell. It is found in the highest amounts in the human liver, with total body stores of 50-120 milligrams (mg). Copper is directly and indirectly needed for thyroid hormone synthesis. It does so indirectly by being a part of the mitochondria’s respiratory chain, which produces the critical energy currency, adenosine triphosphate (ATP). In this case, copper allows the transfer of electrons along the mitochondrial membrane by aiding the cytochrome-c oxidase protein in accepting electrons. The formation of cytochrome-c, copper, and iron-heme are termed Complex IV which is the final electron acceptor where it passes the electrons to molecular oxygen. This final step eventually, with the binding of hydrogen, converts oxygen into water and releases ATP. In addition to cytochrome-c protein function, this mineral is needed for its cellular biogenesis.
Among other processes utilizing copper-mediated reactions are collagen and connective tissue formation, hematopoiesis (red and white blood cell development), and bone formation. Dysregulation of copper metabolism can be observed in genetic disorders such as Menkes syndrome. Not to mention, the reduction of this essential molecule causes abnormal function of the glutathione enzymatic system.
Both selenium and copper are required for optimal function of the antioxidant system, without which a person may experience thyroiditis (inflammation and injury of the thyroid gland). In susceptible populations, sub-optimal selenium and copper cause an increase in potentially damaging oxidants such as superoxide and hydrogen peroxide molecules. As mentioned, there is a need for the glutathione system regulated in part by copper and selenium. Copper is also required for the activity of superoxide dismutase which mediates antioxidant clean up during the synthesis of thyroid hormone production.
The Immune System’s First Line of Defense Requires Copper
To appreciate the importance of copper in forming the immune system consider the case of a woman diagnosed with Myelodysplastic Syndrome (MDS) and severe neutropenia. MDS is a name given to a group of bone marrow failures often related to acquired genetic disorders and cancer. In this case, it was found that a copper deficiency was the culprit behind the diagnosis of MDS. The syndrome, often associated with an impending cancer, was resolved by the repletion of copper. This is not a lone case, as loss of copper is a known cause of hematopoietic failure including anemia or reduction of Red Blood Cells (RBC). Even though the concept of copper deficiency and hematopoiesis can be found in the medical literature, it is commonly not considered by treating physicians.
It is important to understand that the above case occurred with a severe deficiency and represents only tip of the iceberg. A more common chronic mild copper deficiency can eventually reduce the amount of neutrophils – albeit not as much as a severe deficiency. Moreover, even with sufficient number of neutrophils, the reduction of this mineral inside of these cells can reduce their immunological power. Specifically, neutrophils become weak in pulling in bacteria (phagocytosis) as well as killing them inside of their compartments once they are pulled in.
The mechanisms behind why low copper levels induce neutropenia are still elusive and require much research to understand. However, it is proposed that copper can activate certain genes and factors required for neutrophil development. We also cannot ignore that the electron transfer properties of this mineral are required for ATP production. This is paramount for cells with high turnover such as neutrophils. Therefore, we can confidently speculate that any reduction of this energy system, such as in copper depletion, can cause a development disruption in energy-demanding processes.
Copper and Thyroid Health
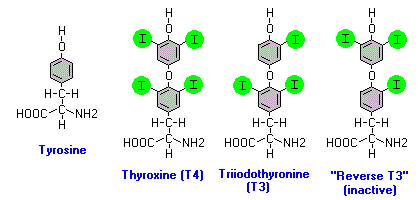
Generally when the word “minerals” cross the mind in context of the thyroid, we tend to think of the essential mineral iodine. Nonetheless, while iodine is part of and therefore crucial for formation of thyroid hormones, Thyroxine (T4) and Triiodothyronine (T3), other minerals and co-factors pave the road for its incorporation into a final hormone form. Among important mediators of this process is copper. We have thus far described how it is indirectly needed to process the energy needed for hormone production through being a vital part of the mitochondrion’s electron transport chain. What is not commonly known or stated is that copper can mediate iodination (or halogenation) reactions.
How does copper directly mediate thyroid hormone production? To answer this question we briefly must first delve into how the thyroid hormones are made. The basic requirements needed here are:
- Iodide
- Tyrosine as a backbone (see image)
- Oxidation chemistry to make molecular iodine from iodide.
Using the above components, the reaction goes in this order:
- Oxidation of iodide into iodine utilizing hydrogen peroxide and thyroid peroxidase.
- Conjugation of the electrophilic iodine species into aromatic rings of tyrosine creating derivatives of thyroxine (i.e. one or more tyrosines with one, two, or more covalently linked iodine atoms).
- The above happens on the back of thyroglobulin inside the thyroid.
Where does copper come into this? Step 2 above requires that iodine molecules become electrophilic and reactive towards the tyrosine aromatic ring. This can happen in the presence of hydrogen peroxide thereby making it very electrophilic. This means that the molecular I2 Iodine reacts as if it was an I+.
However, it is very well known in organic chemistry synthesis that these reactions (i.e. creation of electrophilic iodine) can be mediated in the presence of copper chloride salts (CuCl2). We speculate that this reaction occurs within the tyrosine-base (present in thyroglobulin) in what is called aromatic iodination. Here, copper salts act as a catalyst to iodination of tyrosine. A somewhat similar reaction used in organic chemistry synthesis is called the Sandmeyer Reaction.
What Causes Low Copper and Where Can You Obtain It?
Reduction of copper reserves is more prevalent than is commonly presented in medicine and can be due to a variety of reasons, the simplest of which is attributed to insufficient dietary intakes and/or malabsorption. Take the case of a 17 year old diagnosed with Celiac disease presenting with substantial anemia and neutropenia (see neutrophil section above). This hematopoietic abnormality could not be corrected when the patient was put in an eight month gluten free diet along with other vitamins such as B12 and iron. This abnormality was only corrected after incorporation of a two month copper supplementation regimen. This, similar to the first patient above, describes the importance of this mineral when considering differential diagnosis in anemia and immune dysfunction. Other factors that can cause deficiencies are listed below:
- Excessive zinc intake. There are many reported cases in the medical literature regarding the overuse of zinc without countering or balancing it with copper. Zinc is crucial to immunity, nonetheless too much can deplete copper and causes immune and biochemical imbalance.
- Inflammatory bowl syndrome or Cystic Fibrosis.
- Undergoing a bariatric surgery.
- There is an increased demand during pregnancy and lactation, therefore borderline deficient women may experience overt deficiency symptoms during pregnancy or lactation.
The best natural sources for copper include liver, oysters, and plant nuts/seeds. Liver is optimal because it covers a lot of potential deficiencies (iron, B2, B12, and other factors). However, supplementation can be critical in cases of substantial deficiencies where even liver inclusion would not suffice. As observed in the Celiac disease patient above, they needed two month of copper supplementation in order to resolve the deficiency symptoms. Therefore, under medical supervision, supplementation will be required in cases where deficiency has become chronic with overt symptoms. There are many chelate-based supplements that include copper-glycinate and orotate. For more information on how to determine if one is deficient in this essential mineral we refer the reader to Dr. Chris Masterjohn’s analysis on this aspect.
Copper Is An Essential Nutrient
In addition to copper’s role in the electron transport chain, and antioxidant support in the thyroid, it is also essential for proper immune (neutrophil) function. Treating physicians must consider possible copper deficiency in patients who display neutropenia or MDS type syndromes. This is especially true for patients with relatively higher risk of a copper deficiency such as one with Celiac disease or bariatric surgeries. We also present plausible copper-mediated reactions in the thyroid glands. Similar to what is known in medicine regarding hydrogen peroxide in the thyroid glands, copper is likely important in mediating the aromatic iodination of tyrosine by making iodine highly electrophilic towards the tyrosine rings. This iodination chemistry mediated by hydrogen peroxide and copper salts therefore initiates the process for thyroid hormone formation.
Acknowledgment
I would like to thank Jason Hommel for his scientific discussions and sharing experiences with copper chelates.
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
Image by Alexas_Fotos from Pixabay.
This article was published originally on August 12, 2020.












Glad to see copper being discussed. I think it is just as important as Thiamine. I was unable to tolerate Thiamine or any other B-vitamins for the longest time until I began taking 10 mg of copper per day. Copper has dramatically improved my health along with thiamine.
Thank you for this article.
I had previously tried the high-dose Thiamine (TTFD) Protocol, taking an astronomical 2000mg per day, with the proper co-factors, but had ZERO improvements. However, supplementing with copper, balanced with other minerals and nutrients, has provided more positive results.
I’m grateful this information is finally coming to light.
Balancing minerals can indeed become a puzzle. I am happy that you figured out the need for copper on your protocol. Thank you for sharing your experience, Kimberly!
Can you say how much you took and what you took? I’d love to know to get myself better to. I’ve been doing the thiamine protocol for 70 days now and haven’t seen any results…. Also how long did you try the b1 protocol for and still didn’t see results? Thanks
Not sure if my comment posted or not so, sorry if I’m repeating myself. What’s your protocol with copper?
Women with ancestry and personal experience of major depressive disorder and/or post-parting depression may have difficulty excreting copper. Copper blocks neurotransmitter synthesis.
Hello thats so interesting about the macrocytic anemia that is unresponsive to B12 iron. Is there a big difference between copper sulphate and copper glycinate as far as supplementation? I have a deficiency but I find even at 3mg of copper glycinate I become nauseous. I have read that copper helps the body detoxify fluoride and metals by speeding up thyroid hormone as well as SOD. I’m wondering if the nausea is a need for Iodine support or tyrosine? thank you
Hello Brittany,
SOD is an important member of the body’s antioxidant defense system. It requires both copper and zinc as cofactors in order to quench reactive substances like superoxide radicals. Copper is also needed for ATP production (it is part of the electron transport chain) – in this way it provides energy to the liver and other cells to detoxify and remove unwanted molecules and propagate enzymatic reactions. With regards to the nausea, although it’s too general of a symptoms, if you are taking it on empty stomach then take the copper with food. Low stomach acid may also be a culprit as it is needed for mineral absorption. Since you are taking copper amounts close to the RDA anyways then maybe eating liver, or desiccated liver may be the best for now.
Hi Mahmood!
Many with weird diseases have low ceruloplasmin,
as measured in blood tests.
Supplementing copper might thus be tricky?
Hi KayT!
You are absolutely right. This is why testing in context of clinical history would be the optimal route before commencing supplementation. Testing serum copper along with ceruloplasmin at the same time will give an indication of what the cause may be (i.e. copper deficiency vs a disease like Wilson’s). When suspecting genetic disorders for copper metabolism then addition of urine testing should be indicated and if needed, followed by genetic analyses.
On the other hand, ceruloplasmin can be misleadingly high during an inflammatory state so that must be factored in.
That would be low ceruloplasmin not due to a genetic disorder.
This seems to be common in weird diseases.
hello, how much do you think is the right dosage of copper?
How do you know if you are deficient?
Hi Silvi,
The official RDA for copper hovers around 1-1.5 mg per day. However the upper limit is set at 10 mg per day from all sources. In my opinion I think the RDA needs to be higher. You can get a blood test for serum copper along with ceruloplasmin. You want to be above mid-range for these tests. Note that ceruloplasmin can be misleadingly high if there is an ongoing inflammation, for example. There are other indirect indicators such as microcytic (or even macrocytic) anemia that is not responsive to iron or B12 replacement.
What happens if you know you are deficient? In clinical practice patients who are severely deficient in copper are treated with a combined dose of copper-IV and supplementary copper (8mg per day) for months – though the IV stops within weeks. It may take up to 7 months for the deficiency to completely correct (i.e. clinical symptoms to go away). However, patients progressively experience symptom withdrawal preceded by correction of biochemical markers (anemias, copper levels, etc..). Here is an example case study of a person who had hematological and neurological symptoms from low copper (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2712481/)
Dr. Chris MasterJohn has an excellent explanation on this subject (https://chrismasterjohnphd.com/podcast/2017/02/03/manage-copper-status)