Dexamethasone or DEX, the synthetic corticosteroid that mimics the anti-inflammatory and immunosuppressant effects of endogenous cortisol, has been given to pregnant women who are at risk of delivering a child with congenital adrenal hyperplasia (CAH) for almost 30 years, despite the fact there are no data indicating either its safety or efficacy, and one study from Sweden suggesting such a high risk of adverse events and long term consequences, that the study was halted and the use of the drug was banned.
Similarly without evidence, physicians who specialize in vitro fertilization (IVF) are using DEX to prevent miscarriage. There is only one small and recent study suggesting that DEX may augment ovarian response and increase follicle production for egg retrieval. There are no studies showing improvement in fertilization, implantation or pregnancy rates, or even data showing it prevents miscarriage, its supposed purpose. Indeed, IVF physicians have embraced the myth of this miracle hormone, on perhaps no more than medical hunch.
Dexamethasone and Ovarian Germ Cells
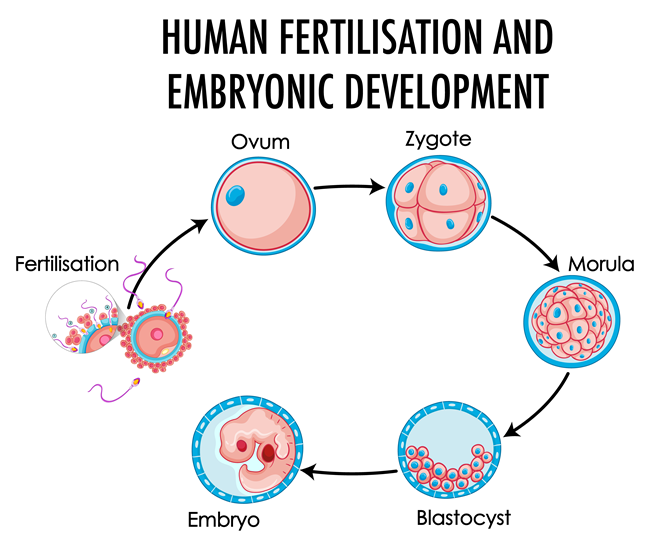
A recently published study looked at the impact of in vitro – organ culture – exposure of fetal ovaries (obtained from recent abortions), and ovary germ cell development. What they found was Dexamethasone Induces Germ Cell Apoptosis in the Human Fetal Ovary. Remember germ cells are those that are handed down at birth from our parents that contain the genetic materials needed to form ovarian follicles (eggs) for women, sperm cells for men. We know that dexamethasone impairs genital development in males, but this is the first study to look at DEX and females – and they went right to the source, germ cell development.
Typically germs cells divide in a logical sequence that eventually results in oocytes or eggs for women or sperm cells for men. In some women and men, the cell division progresses unconventionally, as a result of epigenetic factors including the health and environmental exposures of our parents, even our grandparents. In utero exposures to medications, such as DEX, vaccines and other toxins can cause errors in germ cells. Germ cell division is very highly environmentally influenced and as such, it is not a big leap to think that fetal exposure to synthetic hormones such as DEX during germ cell division – weeks 6-20 of pregnancy, would have an impact on ovarian health. Indeed, it does.
Researchers found that when the fetal ovaries were exposed to dexamethasone in culture for only two weeks, the rate of germ cell death increased, the density or total number of germ cells decreased, as did the expression of one of the genes associated with germ cell survival. This was with only two weeks of exposure. In most cases, women at risk of having a baby with CAH are given dexamethasone continuously from nine weeks through the first trimester. Those pursuing IVF are given DEX preconception through the first 10 weeks of pregnancy, though at a reduced dose compared to CAH. In both cases, fetal exposure to dexamethasone is chronic, during the most critical period of reproductive organ development and germ cell division, a fact that seems to be missed with the purveyors of this drug.
What Happens When We Alter Germ Cell Development?
While there may be limited adverse effects in the moms given dexamethasone, their offspring and potentially even their grandchildren may have varying levels of altered reproductive and sexual development, including changes in the structure and function of the reproductive organs, but also, in the brain chemistry that supports gender identity. We don’t know, however, because there have been so few studies and so little recognition of the potential dangers associated with prenatal dexamethasone, or even with prenatal hormone exposures in general.
In male rodents, exposed to dexamethasone in utero, there are significant problems: reduced penis size, malformed genito-urinary tracts, undescended or malformed testicles and even testicular germ cell cancer. Until this publication, females, animal or human, had not been studied. The fact that they observed germ cell errors, leads one to speculate that later in life, perhaps similar to the DES daughters and granddaughters, these women too will experience the congenital uterine malformations and the complement of reproductive diseases, that include various cancers. At the very least, because of the increased rate of germ cell apoptosis – cell death – observed in the present study, the researchers speculate in utero exposure to dexamethasone will elicit a higher and earlier rate of premature ovarian failure in the offspring.
What becomes abundantly clear is that we ought to stop dosing pregnant women with drugs, especially those hormonal in nature, when we have no data supporting safety or efficacy. We ought to recognize that these substances cross the placental barrier and will affect fetal development. Given medical history over the last 70 years from DES, thalidomide and now, DEX, it is clear any changes in medical practice must be initiated by women themselves. There seems no impetus from medical science to investigate before medicating pregnant women.
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
Image by brgfx on Freepik.