As a child, at around age 5, I started experiencing severe pain in my legs and back. I had migraines from hell that made me feel as if my head were going to explode. I had hair in places that no five-year-old should and I needed a bra for kindergarten. I looked down on all my peers because I was a good 12 inches taller than most of them. I always had to be the mom when playing house in kindergarten. My parents struggled to find help for me because no doctor could explain why I hurt and felt the way I did. Finally, after being hospitalized for over a week my parents had answers. I was diagnosed with a hormone imbalance. The doctors in the area where I lived had no answers about how to treat me, so I was sent to a specialist in Augusta Georgia. He then called my diagnosis precocious puberty, a hormonal imbalance. After more needles and tests, he decided to start me on Lupron.
I took Lupron as a child from 1989 to 1994 for my hormone imbalance. I received a shot once a month in my thigh and sometimes in my butt cheek. I can remember the burn of the Lupron going into my body like it was yesterday. The injection always took me a week to recover from. I experienced soreness in the injection spot and redness every time I received Lupron. I could not run and play as a child. My legs either hurt or my mind would make me feel as though I had no purpose. From the outside, the Lupron shots were a success. The shots slowed my abnormal hair growth in private places and stopped my breast from growing, but on the inside, where it counts, what was the Lupron doing to me?
After being on the Lupron for half a year, I had lumps in my thighs where I received the injections (which I still have today). One time it crippled my legs, leaving me on crutches for two months when I was 7 years old. Yet still, I guess my doctor felt it would be more beneficial for me to continue my treatment with the Lupron because I received injections for 3 to 4 more years.
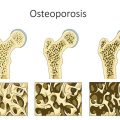
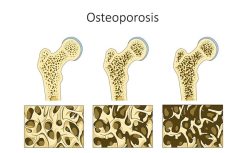
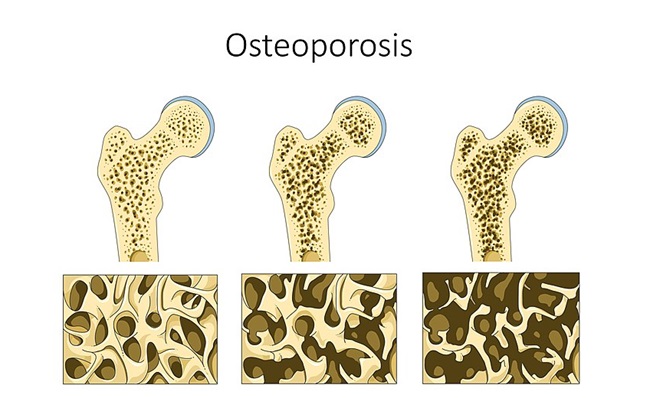
While on Lupron, I struggled daily to focus. I had blurred vision. I battled depression and I hurt daily. I couldn’t be active in sports due to my low bone mass and what kid feels like playing ball when her body feels like it’s been beaten with the bat already? By age 11, I finished up my treatment. I saw my doctor a handful of times after and then I was written off as cured, and I did feel good and enjoyed life for a few years.
At age 15, I began to battle depression. I struggled to remember things. I was diagnosed with asthma. My sight was getting worse and I had an eating disorder where I never wanted food due because my stomach felt like someone was squeezing it with all their might. I fainted several times because I was dizzy. I struggled to stand and I would walk holding onto things. My legs, arms, fingers, and toes would tingle and go numb. My doctor at the time seemed to always just prescribe me Lortabs for the pain and never dug any further to figure out why I felt the way I did. At age 17, I overdosed on Lortabs not trying to hurt myself but only trying to find relief from the pain. I didn’t realize that my body couldn’t handle all the medicine I was putting into it. My mom found me in my room and rushed me to the hospital where my stomach was pumped and I had to drink this disguising chalky drink. My pain seemed to decrease shortly after I turned 20. I looked and felt like the average healthy person. I had my first baby at 21 and my second at age 22. I had healthy pregnancies and healthy babies. I had my third baby at age 32 and again I was a healthy mama and had a healthy baby.
For the past two years, however, I have been experiencing a pain that is all too familiar. I feel as though my body and mind are failing me. I cannot pinpoint why all of this is happening to me. I am now 34 years old and experiencing horrific body aches and pains. I’m searching for answers as I struggle to hold my baby and even get out of bed due to the pain I feel. I hurt in all my joints, my back, my neck and I have leg pains that put me on my knees and unable to walk. My feet and ankles swell, my arms, fingers, and toes tingle with pain. I sometimes drop things because I can’t squeeze my fingers together to hold them. I am experiencing night sweats, hot flashes, nausea, and abnormal weight loss. I have dropped 90 pounds in 15 months. My stomach hurts every day and I never feel hungry. I have so many other symptoms, I feel as though I’m falling apart. I am working with my doctor now to try and find answers. I want to find quality in my life again. I want to feel good when I wake up and not have to lay in bed for at least an hour (waiting for pain meds to kick in) before I can move. Most importantly, I want to be able to pick my baby up out of her crib and hold her and not cry because it hurts my body so bad.
I am in the beginning stages of trying to figure out why I feel this way. I have had blood work drown which was good and I have a CT scan this week. It never even crossed my mind that all of this could be the side effects of taking Lupron as a child until I started reading other people’s stories. Please help?? What test do I need to take to help find answers? What meds are helping restore bone mass? What are other past Lupron patients experiencing and what is working to help them find quality in life again? Thanks in advance for your help!
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
Image by AlexLoban from Pixabay.
This story was published originally on June 18, 2018.