The Threat Around Us
Animals, including Homo Sapiens, survive in an essentially toxic environment, surrounded by microorganisms, potential poisons, the risk of trauma, and adverse weather conditions. Evolutionary development has equipped us with complex machinery that provides defensive mechanisms when any one of these factors has to be faced. Before the discovery of microorganisms, medical treatment had no rhyme or reason, but killing the microorganisms became the methodology. The research concentrated on ways and means of “killing the enemy”, the bacteria, the virus, the cancer cell. The discovery of penicillin reinforced this approach. We are now facing a period of potential impotence because of bacterial resistance, failure of attempts to kill viruses, and the resistance to chemotherapeutic agents in cancer. Louis Pasteur is purported to have said on his deathbed, “I was wrong, it is the terrain that matters”, meaning body defenses.
Hans Selye, whose research into how animals defend themselves when attacked by any form of stress, led to his description of the General Adaptation Syndrome (GAS). He recognized the necessity of energy in initiating the GAS and its failure in an animal that succumbed to stress. He labeled human disease as “the diseases of adaptation”. In Selye’s time, there was little information about energy metabolism but today, its details are fairly well-known. The suggestion of a new approach depends on the fact that our defenses are metabolic in character and require an increase in energy production over and above that required for homeostasis. If the GAS applies to human physiology and that we are facing the “diseases of adaptation”, it is hypothesized that research should be applied to methods by which energy metabolism can be stimulated and mobilized to meet the stress.
Energy Deficiency, Defective Immunity, and COVID-19
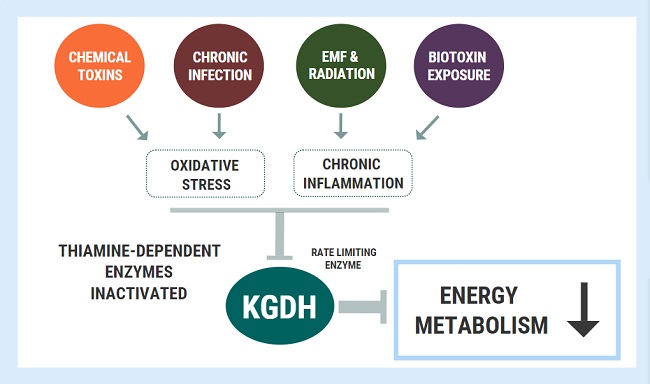
There is evidence that energy deficiency applies to each of the diseases described here. It may be the unrecognized cause of defective immunity in Covid-19 disease. Although in coronavirus disease the clinical manifestations are mainly respiratory, major cardiac complications are being reported involving hypoxia, hypotension, enhanced inflammatory status, and arrhythmic events that are not uncommon. Past pandemics have demonstrated that diverse types of neuropsychiatric symptoms, such as encephalopathy, mood changes, psychosis, neuromuscular dysfunction, or demyelinating processes may accompany acute viral infections or may follow infection by weeks, months, or longer in viral recovered patients. Electrocardiographic changes have been reported in Covid-19 patients. The authors suggest that it may be attributed to hypoxia as one possibility. Because the total body stores of thiamine are low, acute metabolic stress can initiate deficiency. Thiamine deficiency has a clinical expression similar to that observed in hypoxic stress and the authors referred to it as pseudo-hypoxia. It is therefore not surprising that defective energy metabolism can express itself clinically in many different ways.
The present medical model regards each disease as having a separate cause, but the large variety of symptoms induced by thiamine deficiency suggest the ubiquitous nature of energy deficiency as a cause in common. Obesity, a reflection of high calorie malnutrition, has been published as a risk factor for patients admitted to intensive care with Covid-19. Thiamine deficiency was reported in 15.5-29% of obese patients seeking bariatric surgery. Hannah Ferenchick M.D. an emergency room physician commented online that many of her patients with Covid-19 had what she called “silent hypoxemia”. These patients had an arterial oxygen saturation of only 85% but “looked comfortable” and their chest x-rays “looked more like edema” It has long been known that patients with beriberi had low arterial oxygen and a high venous oxygen saturation. All that would be needed to support the hypothesis of thiamine deficiency in some Covid victims would be finding a high venous oxygen saturation at the same time as a low arterial saturation. Also, edema is a very important sign of beriberi, and thiamine deficiency has been noted in critical illness.
Disrupted Autonomic Function
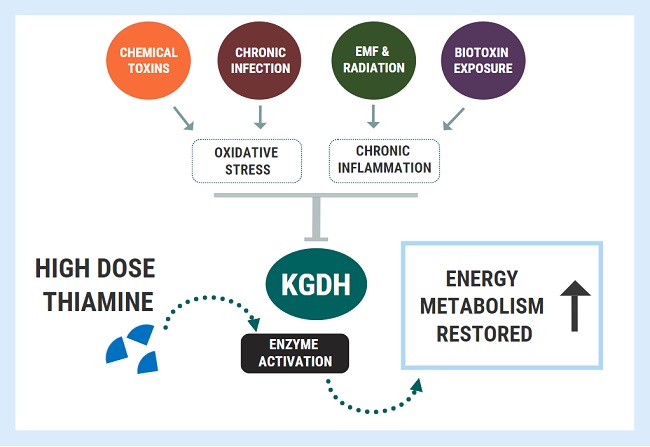
There have been many articles in medical journals describing dysautonomia, mysteriously in association with a named disease, but with no suggestion that the dysautonomia is part of that disease. More recently, there is increasing evidence that dysautonomia is a feature of chronic fatigue syndrome (CFS), manifested primarily as disordered regulation of cardiovascular responses to stress. Manipulating the autonomic nervous system (ANS) may be effective in the treatment of CFS. Dysautonomia is also a characteristic of thiamine deficiency. Patients with Parkinson’s disease begin to lose weight several years before diagnosis and a study was undertaken to investigate this association with the ANS. Costantini and associates have shown that high dose thiamine treatment improves the symptoms of Parkinson’s disease, although the plasma thiamine concentration was normal. They have also shown that high dose thiamine treatment decreases fatigue in inflammatory bowel disease, Hashimoto’s disease, after stroke, and multiple sclerosis. As already noted, it is also an important consideration in critically ill patients.
Multiple System Atrophy is a devastating and fatal neurodegenerative disorder. The clinical presentation is highly variable and autonomic failure is one of its most common problems. Dysautonomia was found to be a clinical entity in Ehlers-Danlos syndrome, a musculoskeletal disease, and this syndrome frequently coexists with Postural Orthostatic Tachycardia Syndrome (POTS), a disease that is included in the group of diseases under the heading of dysautonomia. Some cases of POTS have been reported to be thiamine deficient. This common condition often involves chronic unexplained symptoms such as inappropriate fast heart rate, chronic fatigue, dizziness, or unexplained “spells” in otherwise healthy young individuals. Many of these patients have gastrointestinal or bladder disorders, chronic headaches, fibromyalgia, and sleep disturbances. Anxiety and depression are relatively common. Not surprisingly the many symptoms are often unrecognized for what they represent and the patient may have a diagnosis of psychosomatic disease.
Immune-Mediated Inflammatory Diseases (IMIDs) is a descriptive term coined for a group of conditions that share common inflammatory pathways and for which there is no definite etiology. These diseases affect the elderly most severely with many of the patients having two or more IMIDs. They include type I diabetes, obesity, hypertension, chronic pulmonary disease, coronary heart disease, inflammatory bowel disease, rheumatoid arthritis, Sjogren’s syndrome, systemic lupus, psoriasis, psoriatic arthritis, and multiple sclerosis. The recent recognition of small fiber neuropathy in a large subgroup of fibromyalgia patients reinforces the dysautonomia-neuropathic hypothesis and validates fibromyalgia pain. These new findings support the disease as a primary neurological entity.
Energy Deficiency During Pregnancy: The Cause of Many Complications
Irwin emphasized the energy requirements of pregnancy in which the maternal diet and genetics have to be capable of producing energy for both mother and fetus. He found that preventive megadose thiamine, started in the third trimester, completely prevented all the common complications of pregnancy. Hyperemesis gravidarum is the most common cause of hospitalization during the first half of pregnancy and is second only to preterm labor for hospitalization in pregnancy overall. This disease has been associated with Wernicke’s encephalopathy, well known to be due to brain thiamine deficiency. The traditional explanation is that vomiting is the cause, but since vomiting is a symptom of thiamine deficiency, it could just as easily be the cause rather than the effect. In spite of the fact that migraines are one of the major problems seen by primary care physicians, many patients do not obtain appropriate diagnoses or treatment. Migraine occurs in about 18% of women and is often aggravated by hormonal shifts. A complex neurological disorder involving multiple brain areas that regulate autonomic, affective, cognitive, and sensory functions, it occurs also in pregnancy. Features of the migraine attack that are indicative of altered autonomic function include nausea, vomiting, diarrhea, polyuria, eyelid edema, conjunctival injection, lacrimation, nasal congestion, and ptosis.
The Proteopathies: Disorders Involving Critical Enzymes
The earliest and perhaps best example of an interaction between nutrition and dementia is related to thiamine. Multiple similarities exist between classical thiamine deficiency and Alzheimer’s disease (AD), in that both are associated with cognitive deficits and reductions in brain glucose metabolism. Thiamine-dependent enzymes are critical components of glucose metabolism that are reduced in the brains of AD patients. Senile plaques and neurofibrillary tangles are the principal histopathological marks of AD and other proteopathies. The essential constituents of these lesions are structurally abnormal variants of normally generated proteins (enzymes). The crucial event in the development of transmissible spongiform encephalopathies is the conformational change of a host-encoded membrane protein into a disease associated, fibril forming isoform. A huge number of proteins that occur in the body have to be folded into a specific shape in order to become functional. When this folding process is inhibited, the respective protein is referred to as being mis-folded, nonfunctional, and causatively related to a disease process. These diseases are termed proteopathies and there are at least 50 different conditions in which the mechanism is importantly related to a mis-folded protein. Energy is required for this folding process. Because of their reported relationship with thiamine, it has been hypothesized that mis-folding might be related to its deficiency on an energy deficiency basis.
It All Comes Down to Energy
A hypothesis has been presented that the overlap of symptoms in different disease conditions represents cellular energy failure, particularly in the brain. If this should prove to be true, the present medical model would become outdated. An attack by bacteria, viruses or an oncogene might be referred to as “the enemy”. The defensive action, organized and controlled by the brain, may be thought of as “a declaration of war” and the illness that follows the evidence that “a war is being fought”. This concept is completely compatible with the research reported by Selye. It underlines his concept that human diseases are “the diseases of adaptation”, dependent on energy for a successful outcome in a “war” between an attacking agent and the complex defensive actions of the body. Killing the enemy is a valid approach to treatment if it can be done safely. Unfortunately, the side effects of most medications sometimes makes things worse and that is offensive to the Hippocratic Oath. We badly need to create an approach to research that explores ways and means of supporting and stimulating the normal mechanisms of defense.
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
This article was published originally on May 11, 2020.