What could male astronauts possibly have in common with earthbound women who use progestin based birth control? On the surface, absolutely nothing, but it turns out that space travel and IUDs (and other medications) initiate some chemistry changes that result in significant neuro-ocular damage. The syndrome, variously called pseudo-tumor cerebri, intracranial hypertension or in the case of the astronauts, space flight associated neuro-ocular syndrome (SANS), compresses and deforms the optic nerve, leading to varying degrees of vision loss and, when severe enough, can lead to blindness. On earth, it affects predominantly overweight women of childbearing age, many of whom use progestin based birth control (Mirena IUDs and the like). Fully 90% of the terrestrial cases are female and anywhere from 70-88% depending upon the study population, are considered obese. In contrast, those affected during space travel are predominantly male, of normal weight, and generally, quite healthy. Different conditions right? Maybe, but I don’t think so.
On the Surface: Pseudotumor Cerebri, Intracranial Hypertension and SANS
Pseudotumor cerebri (PTC) and intracranial hypertension (IH) refer to conditions whose predominant symptom is increased cerebral spinal fluid (CSF) pushing against the optic nerve. The condition is said to begin with a severe headache that is worse while laying down. The head pain is accompanied by vision changes or vision loss, and sometimes also synchronous pulsatile tinnitus (pulse based ringing in the ears described as a whooshing sound), and nausea. If sufficient intracranial pressure builds up, other symptoms may develop including sharp nerve pain in the arms, legs, and back, severe neck stiffness, numbness or tingling in hands, feet, and face, depression, exercise intolerance, and memory difficulties. These symptoms occur absent a tumor, and hence, the name pseudo or false tumor.
In contrast, SANS appears to develop without the severe headache. Microgravity plays some interesting tricks on fluid dynamics (and our ability to measure it), however, and so it is not clear whether elevated intracranial pressure can develop in microgravity and/or if it does develop, is offset by other changes in pressure dynamics. One short term study of acute changes in pressure during the simulated microgravity of a prolonged head down tilt test and a 24 hour parabolic flight, concluded that intracranial hypertension does not exist during space flight. It should be noted, that even during the short exposure to microgravity in this particular study, intracranial pressure did increase over what is observed on earth. It simply did not increase to the degree seen in intracranial hypertension. Moreover, how changes during 24 hours of microgravity accord with the typical 6-12 month duration space flight where SANS is typically observed, remains to be elucidated.
Despite the question of whether or not intracranial hypertension exists in space, both SANS and its earthbound counterparts inevitably result in the same neuro-ocular deformations. These include: choroidal folds, optic disc edema, cotton-wool spots, globe flattening, and refraction changes. So here we have two conditions, affecting completely different populations, with descriptive diagnoses that focus on what are perceived to be the predominant manifestations of the disease process. In women, the focus is on the increased intracranial pressure that seemingly results in optic nerve damage, while in the astronauts, the focus is on the optic damage either absent intracranial hypertension or preceding it. Conceivably, in both conditions optic nerve damage begins long before sufficient pressure changes evoke head pain. That is, there may be a time course to this process wherein slight changes in pressure exert damage long before the process is fully fomented and the other symptoms emerge. The time course may be such that recognition and attribution of symptoms occurs late in women but early in male astronauts, if only because researchers are looking more closely at the astronauts.
Potential differences in time course aside, could these two presumably discrete conditions, developing in such widely disparate populations, share other commonalities? Yes, and interestingly enough, one of the primary threads leads us to hormones and nutrients of all places. Sure, it was obvious that hormones would be involved in the IUD induced cases, but in male astronauts too? Yep.
The Nitty Gritty: Altered Steroidogenic Enzymes
It turns out that there is a good chance that steroidogenic enzymes responsible for metabolizing glucocorticoid and mineralcorticoid hormones and regulating salt/water balance in both the kidneys and the choroid plexus (the part of the brain where spinal fluid is produced and released) are upregulated in folks who develop these conditions.
Terrestrial Women
In the women, the process can be initiated by either the progestin (levonorgestrel) released by the IUD, implant, shot, or by excessive weight. Both the continuously high dose of levonorgestrel released by the IUD and excessive weight appear to independently, and likely synergistically when combined, force a shift in the downstream enzymes to compensate. In the case of the IUD, many of the enzymes along the steroidogenic pathways are re-regulated because of the progestin. For example, 17B-HSD2 (17 beta hydroxysteroid dehydrogenase type 2), the enzyme responsible for converting estradiol to the less potent estrone and testosterone to the less potent androstenedione is downregulated. This means that women who use these IUDs may have higher testosterone concentrations, which would then produce a feedback loop linked to insulin resistance and other metabolic changes that also change kidney electrolyte balance. If the woman is overweight to begin with and/or has polycystic ovarian syndrome (PCOS), testosterone concentrations were already likely elevated and will be exacerbated.
Another enzyme further down the pathway is also upregulated, 11B-HSD (11 beta hydroxysteroid dehydrogenase) and this is the important one for our purposes. 11B-HSD controls cortisol and aldosterone concentrations. Hyperactive 11B-HSD results in altered cortisol and autonomic response and elevated aldosterone concentrations (sometimes called primary, secondary or idiopathic aldosteronism). Elevated aldosterone skews salt/water balance in the kidneys, and most notably, in many instances will increase blood pressure, but may also, and in some cases, disrupt intracranial pressure. Elevated 11B-HSD has been noted with obesity in adult women, men, and children, but also, with intracranial hypertension. Similarly, both secondary aldosteronism and hypertension have been noted with progestin-only IUDs and in male astronauts.
Male Astronauts
Both male and female astronauts appear to develop SANS at almost equally high rates, however, most of the space flights and much of the subsequent research includes only or mostly males. Moreover, in the few instances that included female astronauts, data regarding birth control were not given. So the question is, how do very healthy, normal weight, male astronauts develop a condition that is so commonly associated with a progestin only IUD and obesity in earthbound women? Again, part of the answer points to hormones, or more specifically, the re-regulation of hormones, enzymes, and the like, relative to zero gravity.
Among the endocrine adaptations that occur relative to space include those involved in regulating fluid and electrolyte balance, water/salt homeostasis in the kidneys and elsewhere. Specifically aldosterone, renin and angiotensin hormones increase during space flight resulting in a sort of secondary aldosteronism, just like we saw in the terrestrial women. Only in the male astronauts, it is not in response to synthetic hormones or weight related metabolic adaptations but instead is relative to gravity related adaptations. Whether the 11B-HSD enzyme is upregulated is not clear, as it has not been measured, but I suspect it would be.
The re-regulation of 11B-HSD, I believe, is the common thread between the evolution of this disease process in these two distinct populations. There are still more questions to be answered, however. First, what does kidney salt/water balance have to do with fluid balance in the choroid plexus of the brain, and second, neither all earthbound, obese, or IUD using women nor all astronauts develop this condition, what else is involved? There has to be another set of triggers. Indeed, there is.
Fluid and Electrolyte Balance in the Kidneys and the Brain
In one of the more astute papers on this condition, researchers investigating pediatric pseudotumor cerebri, posited regulation of fluid balance in the kidneys occurs via analogous mechanisms in the choroid plexus of the brain. This makes sense given the brain can be considered an endocrine organ, containing the full complement of hormones, enzymes and receptors. When we think about it, why wouldn’t brain endocrine capacity be similar to that of the body? Biological systems are conserved and repeated. Indeed, the choriod plexus, the cavity where CSF dynamics are controlled, is home to some of the same transporters, enzymes, receptors as the kidneys.
From this recognition, those same researchers implicated upregulated 11B-HSD enzymes as one of the key factors in pediatric cases. I should note, obesity plays a role in childhood intracranial hypertension as well, though to what degree, is not clear. Nevertheless, the recognition of upregulated 11B-HSD in pediatric cases suggests that we are on the right track with the women and the astronauts. Now we have three entirely different populations with altered brain pressure dynamics resulting in neuro-ocular damage, all mediated by altered steroid enzyme function. The only question that remains is why some folks are able to withstand these changes and others are not. In other words, if the IUDs, obesity, and zero gravity all upregulate 11B-HSD, and evidence suggests that may be the case, why do only a percentage these people have problems with intracranial pressure and develop the neuro-ocular symptoms?
Enter Nutrients. Yes, Nutrients.
Health is all about adapting to a hostile environment. How well we adapt determines whether we maintain health or become ill. From that perspective, it is not difficult to imagine that what distinguishes how and to what degree illness is expressed might come down not to genetics per se, but to the selective expression of those genetic patterns. Expression of disease may rest on only two factors: stressors and nutrients and the relative balance between them.
In the case of the women who develop intracranial hypertension, the stressors are obvious. Weight or more specifically, the metabolic adaptations that ensue, is one set of stressors. The high dose of synthetic progestin is the other. In the male astronauts, weightlessness is the stressor. In each population, even though the stressors are highly discrepant, the resultant changes in fluid regulation appear to be the same. That is, in these instances, the stressor(s) lead to common compensatory reactions: hyper-aldosteronism mediated by upregulated enzymes that changes fluid dynamics not only in the kidneys but also in the brain.
There is one more piece to this puzzle, however, because not all folks with elevated aldosterone develop intracranial hypertension, neither do all obese and/or progestin-IUD using women, nor do all astronauts develop intracranial hypertension or the subsequent neuro-ocular changes associated with SANS. Some folks adapt to these stressors more successfully than others. Why is that? Well, if you’ve read my work, you might guess, it all comes down to mitochondria and B vitamins. Yes, my favorite mechanisms seem to determine yet another disease process.
NASA and the B Vitamins: The Beginning of a Beautiful Relationship
Here is where it gets really interesting. NASA researchers investigating the SANS, assessed genetic polymorphisms called SNPs that limit B vitamin absorption or metabolism, as well as vitamin (B2, B6, and B12) and hormone status (androgens only, unfortunately) and connected those variables to the likelihood of developing the SANS. The reasoning behind this work was that alterations in what is called 1-carbon metabolism affects homocysteine and androgen levels and those variables are then associated with the development and progression of SANS. This makes sense, as these and other B vitamins are incredibly important to maintaining all sorts of enzymatic functions, not the least of which include those related to mitochondrial functioning.
In measuring the genetic alterations in enzymes involved in B-vitamin metabolism, homocysteine and actual concentrations of B vitamins, before, during and after space flight, the researchers found that higher pre-flight homocysteine (an indication of problems with the methylation pathway), along with a combination of certain SNPs (MTRR-66 and SHMT1) and lower vitamin status (folate, B6 in particular) predicted SANS and the subsequent visual deterioration. Interestingly, higher pre-flight DHEA and in-flight testosterone response was also indicative of SANS, though less significantly with different combinations of androgen response and SNPs predicting specific types of ocular damage.
You’re probably thinking, ‘now wait a minute, how the heck does this connect to hyperactive 11B-HSD, altered aldosterone and the elevated intracranial pressure observed in women?’ Well, for that we have to return to the pediatric cases and unpack a few more details. Bear with me. We’re almost there.
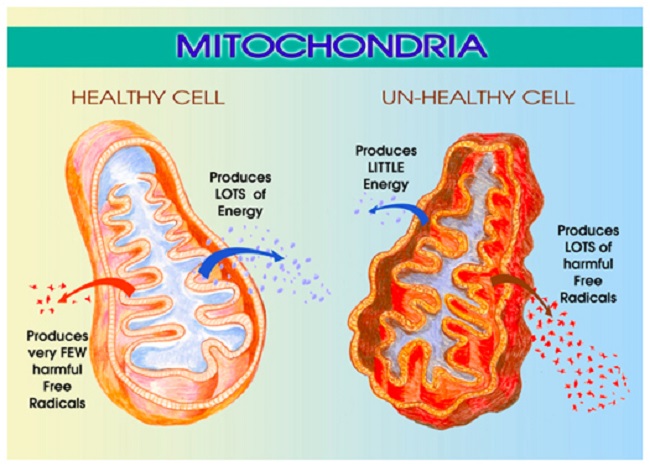
While the NASA studies were impressive, they were somewhat limited in scope. Ideally, a broader range of SNPs, nutrients, and hormones could have been considered. Neither aldosterone nor the other B vitamins involved in mitochondrial functioning were measured in this study. Nevertheless, they provide important clues that when added to those of pediatric cases present a fairly clear picture of disturbed mitochondrial function. These telltale signs of mitochondrial issues like elevated lactate, elevated glutamate and succinate and a disturbed tricarboxylic acid (TCA) cycle (also called the Krebs cycle), would inevitably influence one’s ability to respond to stressors. And they are all thiamine responsive.
Remember from high school chemistry (and all of our posts on mitochondria), dietary nutrients are processed through the TCA cycle, to produce the cellular energy known as adenosine triphosphate or ATP for short. ATP is required for proper cell function. Each of the enzymes within this cycle require vitamins and minerals as co-factors (22 of them in fact). Without these nutrients, the enzymes simply do not work effectively, no matter how much carbohydrate, fat, protein macronutrients are ingested. When the TCA cycle is struggling, energy output is reduced, the ability to detox is reduced, the amount of damaging reactive oxygen species (ROS) is increased and the metabolic balance is shifted.
From NASA, we know that folate and vitamin B6 deficiencies were predictive of SANS and both are indicative of defunct mitochondria. Folate deficiency often means thiamine deficiency and thiamine deficiency means reduced ATP, increased ROS and a cascade of damaging compensatory reactions. Similarly, B6 deficiency equals excess glutamate and from the pediatric paper, we find that glutamate is elevated with intracranial hypertension. Glutamate is a metabolic product of mitochondrial reactions. It is excitatory in nature, and thus, must be kept in balance lest it over- excite cells and induce what is called excitotoxicity, a particularly messy form of cell death. Glutamate just so happens to be in equilibrium with a-ketogluterate dehydrogenase, an enzyme within the TCA that is highly thiamine dependent. Finally, when thiamine is deficient, the enzymes that modulate succinate, another product in the TCA cycle, may also be out of balance. In fact, even though the succinate enzymes are not thiamine dependent themselves, thiamine deficiency, nevertheless, derails the enzymes responsible for deactivating succinate. With elevated succinate, we get yet another link to elevated intracranial pressure and neuro-occular damage. And, as it turns out, elevated succinate leads to activation of the renin-angiotensin-aldosterone system in mice. Since succinate receptors are located in retinal ganglion, we have a direct link from vitamin deficiency (whether by genetic and/or nutritional means), the aldosterone system and ocular nerves.
Tied Up in a Bow
So there you have it: disparate populations exposed to entirely different stressors that result in elevated aldosterone concentrations by compensatory upregulation of the 11B-HSD enzymes, a shift in electrolyte balance in both the kidneys and in the brain, and because of vitamin deficiencies, mitochondrial dysfunction, which further and directly upregulate aldosterone in the ocular nerves inducing damage. A cool set of connections, if I do say so myself.
A few important points to consider. Firstly, what determines who succumbs to any given stressor and how that stressor manifests as a particular disease process rests entirely on the interplay between genes and nutrition. More specifically, adaptive capacity appears to involve the B vitamins and mitochondrial integrity. Secondly, our descriptive process of identifying diseases, while useful as one begins to identify patterns, falls short unless the core mechanisms of the disease process are identified. Arguably, many diseases we have identified as discrete entities are no more than individual manifestations of the same process. In the cases of intracranial hypertension/pseudotumor cerebri and astronaut ophthalmic syndrome/space flight associated neuro-occular syndrome or SANS, though discrete populations and disparate triggers, these conditions are likely all attributable to the same underlying mechanisms: nutrient enzyme mutations, nutrient availability and stressors that preferentially affect salt/water balance via the 11B-HSD enzyme.
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
Image credit: NASA, Astronaut Michael Edward Fossum, Public domain, via Wikimedia Commons
This article was first published December 17, 2017.












Hi Dr Marrs, fascinating to see that this article, written just before the pandemic, apparently has a connection to Covid, in the form of the enzyme you discuss, 11B-HSD (11 beta hydroxysteroid dehydrogenase):
https://twitter.com/boretrol/status/1697454857437999284
You say it alters (reduces?) the concentration of cortisol. I wonder if this could explain the observation of reduced cortisol in Covid that was widely reported:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9519365/
And since you linked your findings to thiamine deficiency, does this provide yet more evidence of a connection between Covid and thiamine deficiency?
(Correction – written two years before the pandemic.)
Hi, I have been taken allthimine ( fat solusble thiamine) After taking it for a few months along with having a copper iud ( non hormonal) i now have intercranial hypertension. I also have gained weight. Any thoughts?
Do you still have the copper iud? Are you taking other medications? Other supplements?
Hi, I have idiopathic intercrainial hypertension. I am overweight now but this started when I was skinny.
I had 1 depo shot in my early 20s that messed me up pretty badly.
I wish I understood this article better. Would thiamine and b supplements help?
Do you know any endocrinologists in Hawaii you would recommend? I also have an empty sella, I’m hoping to find a dr who understands this stuff.
The last one I saw ran the basic labs and then told me I’d be fine if I start walking 5 miles a day.
Abby, I am afraid that I do not know practitioners in Hawaii. Would thiamine help, absolutely. We have dozens of articles on thiamine, not necessarily related to IH but to everything else and to mitochondria. We also have a book on the topic. It’s advertised on the website. I would suggest reading up on thiamine and mitochondrial illness/health in general. You’ll see that the mitochondria control everything, including hormones and inflammation and poorly functioning mitochondria lead to a vast array of symptoms.
Hi Abby,
I’m also dealing with IIH after just one depo shot. The depo shot really has caused me an enormous amount of issues. Did you end up taking thiamine and did it help?
Dr. Marrs and Dr. Lonsdale, What are your thoughts about thiamine used for Idiopathic Intracranial Hypertension (IIH) as a natural carbonic anhydase inhibitor, thus reducing cerebrospinal fluid production?
https://www.ncbi.nlm.nih.gov/m/pubmed/22145674/
https://www.ncbi.nlm.nih.gov/pubmed/21332948
Jason,
I think thiamine is valuable for any number of reasons, not the least of which, it keeps the mitochondria running. We’ve had some success treating IIH with thiamine, but there are often other issues as well and it takes some time for things to unwind. It is not something that will reduce CSF immediately, but over time and along with other nutrients. And in the case of women, with IUDs, the removal of the IUD.
Thank you for this thread. I’m 24 I got IIH a year ago after taking doxycycline for a MRSA infection and I’ve never been the same since. I’ve developed POTS and I still probably have high pressure because my optic nerves are swollen. I would love to know how doxycycline caused this? And what to do about it? I was perfectly healthy until then!
Have you tried taking thiamine?
where do you get an erythrocyte transketolase test??? i cant find one anywhere!!
No where in the US, I am afraid. It is available in London via this lab. http://www.biolab.co.uk/index.php/cmsid__biolab_test/Vitamin_B1_(Thiamine)_-_transketolase_activity
If all this is written for the lay reader, most of it will be incomprehensible and the punchline will be missed. Appropriate nutrition, emphasizing the importance of the B vitamins, may not be enough in today’s toxic world. I am becoming more convinced every day that supplementary water soluble vitamins are becoming a necessity to maintain a reasonable state of health.
I believe accutane causes dysautomia and Thiamine deficiency…..
Retinoic acid = decreased Transketolase (thiamine deficiency)
Accutane inhibits hippocampal neurogenesis by using up the NADPH reducing cofactor… Thiamine
NADPH needed as a CYP26A cofactor for retinoic acid detoxification.
NADPH (B1) and FAD (B2) cofactors should be promoted.
Proteomic approach reveals novel targets for retinoic acid-mediated therapy of thyroid carcinoma.
Our previous studies demonstrated that retinoic acid (RA)-induced reduction of both, the key glycolytic enzyme ENO1 and proliferation-promoting c-Myc, resulted in decreased vitality and invasiveness of the follicular thyroid carcinoma cell lines FTC-133 and FTC-238. By employing two-dimensional electrophoresis and mass spectrometry, we identified proteins affected by RA treatment. In addition to previously reported decrease in ENO1 expression, we found that RA led to significantly reduced levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), pyruvate kinase isoenzymes M1/M2 (PKM1/M2), peptidyl-prolyl cis-trans isomerase A (PPIA), transketolase (TKT), annexin A2 (ANXA2), glutathione S-transferase P (GSTP1) and peroxiredoxin 2 (PRDX2) as compared to untreated control.
https://www.ncbi.nlm.nih.gov/pubmed/20538039
Decreased transketolase activity contributes to impaired hippocampal neurogenesis induced by thiamine deficiency.
Thiamine deficiency (TD) impairs hippocampal neurogenesis. However, the mechanisms involved are not identified. In this work, TD mouse model was generated using a thiamine-depleted diet at two time points, TD9 and TD14 for 9 and 14 days of TD respectively. The activities of pyruvate dehydrogenase (PDH), alpha-ketoglutamate dehydrogenase (KGDH), glucose-6-phosphate dehydrogenase (G6PD), and transketolase (TK), as well as on the contents of NADP(+) and NADPH were determined in whole mouse brain, isolated cortex, and hippocampus of TD mice model. The effects of TK silencing on the growth and migratory ability of cultured hippocampal progenitor cells (HPC), as well as on neuritogenesis of hippocampal neurons were explored. The results showed that TD specifically reduced TK activity in both cortex and hippocampus, without significantly affecting the activities of PDH, KGDH, and G6PD in TD9 and TD14 groups. The level of whole brain and hippocampal NADPH in TD14 group were significantly lower than that of control group. TK silencing significantly inhibited the proliferation, growth, and migratory abilities of cultured HPC, without affecting neuritogenesis of cultured hippocampal neurons. Taken together, these results demonstrate that decreased TK activity leads to pentose-phosphate pathway dysfunction and contributes to impaired hippocampal neurogenesis induced by TD. TK and pentose-phosphate pathway may be considered new targets to investigate hippocampal neurogenesis.
https://www.ncbi.nlm.nih.gov/pubmed/19686241
13-cis Retinoic acid (accutane) suppresses hippocampal cell survival in mice.
Sakai Y1, Crandall JE, Brodsky J, McCaffery P.
Author information
Abstract
Use of the acne drug Accutane (13-cis retinoic acid, [13-cis RA]) has been associated with severe depression. This association has been considered controversial because no causative link has been found between 13-cis RA and this disorder. A recent hypothesis has suggested that atrophy of the hippocampus can result in depression. We now show, in a mouse model, that endogenous RA generated by synthetic enzymes in the meninges acts on hippocampal granule neurons, and chronic (3-week) exposure to a clinical dose of 13-cis RA may result in hippocampal cell loss. In humans this may be conjectured to be the mechanism by which Accutane contributes to depression.
also..
http://www.accutaneaction.com/Studies/2004_Sakai.pdf
Dear Dr. Lonsdale,
I recently discovered your work and everything you explain with such clarity – both on this forum as well as in your books, including the latest one with Dr Marrs – really resonated with me. I’m not a medical doctor but have a PhD in cell biology and have worked for many years in academic research so can (almost always) follow your biochemical explanations pretty closely.
I’ve struggled for several years with the notion of whether supplements are really necessary when one follows a balanced ‘diet‘ (and of course what I mean here is simply eating Mother Nature’s food – not some fad diet). Given the current state of our agriculture however, I’ve also become more and more convinced that even natural foods simply cannot anymore supply sufficient nutrients, and have turned to daily supplementation – B-complex, magnesium, selenium, and zinc. And since discovering your work, 50 mg allithiamine daily as well.
However, I’m also concerned with some of the data out there suggesting that regular supplementation (in absence of disease) could possible act as a trigger for cell transformation or supporting malignant cell growth, down the line. What are your thoughts on this? is there a risk in daily supplementation in the absence of a known deficiency?
It is, without a doubt, Our Toxic Planet! And we take it all the way down to the DNA level, man.
Ironic, what we learn about us on planet earth from the wasteful Space (sic) research (sic) programs. Yet, the fact we are a country — globe — of more and more chronic illness speaks to all sorts of issues:
The following quote from Julian Cribb’s Surviving the 21st Century (Springer Int’l Publishing, Switzerland 2017) likely tells the story:
“The evidence that we ourselves— along with our descendants, potentially for the rest of history— are at risk from the toxic flood we have unleashed is piling up in literally tens of thousands of peer-reviewed scientific research reports. Despite this mass of evidence, the public in most countries is only dimly aware, or even largely unaware of what is being done to them. The reason is twofold: First, most of these reports are buried in scientific journals, written in the arcane and inaccessible language used by specialists. The public may hear a little about certain chemical categories of concern, like pesticides and food additives, or the ‘dirty dozen’ (Stockholm Convention 2013 — I’ll list those below**) industrial super-poisons, or ‘air pollution’ in general. However, these represent only a scant few pixels in a much larger image now amassing in the scientific literature of tens of thousands of potentially harmful substances which are disseminating worldwide. Second, the proportion of chemicals which have been well-tested for human safety is quite small…” (Page 108)
How to put two and two million together, Chandler, in order to get a handle on all the origins of our chronic bad health and the actors involved.
https://www.rand.org/blog/rand-review/2017/07/chronic-conditions-in-america-price-and-prevalence.html
RAND researchers used data from a national survey on health care expenditures to compile a chartbook with the most up-to-date numbers on the cost and prevalence of such chronic conditions. Their estimates suggest that nearly 150 million Americans are living with at least one chronic condition; around 100 million of them have more than one. And nearly 30 million are living, day in and day out, with five chronic conditions or more.
Those at the highest end of the scale, with five or more conditions, represent about 12 percent of the U.S. adult population, but account for more than 40 percent of U.S. health spending, the RAND study showed.
And those oh so sexy rockets getting more junk and junk science into space:
At the same time, the number of launches—for purposes of exploration, tourism, and space-based solar power (PDF)—is expected to increase. One of the study’s co-authors has been quoted as saying, “If left unregulated, rocket launches by the year 2050 could result in more ozone destruction than was ever realized by CFCs.”
https://www.sciencedaily.com/releases/2009/03/090331153014.htm
The global market for rocket launches may require more stringent regulation in order to prevent significant damage to Earth’s stratospheric ozone layer in the decades to come, according to a new study. Future stratospheric ozone losses from unregulated launches will eventually exceed ozone losses from CFCs.
Eat your leafy vegetables and get your daily dose of perchlorate!
Tests on 22 Northern California grocery store samples found perchlorate, a rocket fuel ingredient, in four lettuce samples — though the chemical wasn’t unequivocally linked to Colorado River-irrigated crops.
“The fact that this is now turning up in things that we eat really is an argument for doing as much as we can as fast as we can,” said David Miller, spokesman for state Sen. Nell Soto, D-Ontario, who recently introduced legislation to track and clean up perchlorate contamination in California. “It clearly poses a growing threat not just to California, but to the rest of the country.”
Texas Tech University, which analyzed samples purchased in January and February, found an average of 4 micrograms of perchlorate per 2-ounce serving in the contaminated samples. That would be about four times the amount deemed safe for a liter of drinking water, according to draft health standards by the U.S. Environmental Protection Agency.
State and national drinking water standards for perchlorate are being developed and will account for perchlorate exposure in humans from other sources such as food. In general, the more perchlorate found in the food chain, the lower the regulatory standard will be for drinking water.
In “Suspect Salads,” the Environmental Working Group estimates that by eating lettuce, 1.6 million American women of childbearing age — the population of greatest concern — are exposed daily during the winter months to more perchlorate than the EPA’s interim safe dose recommendation for water.
Eating lettuce or other vegetables grown in fields irrigated by the Colorado River may expose consumers to a larger dose of toxic rocket fuel than is considered safe by the U.S. Environmental Protection Agency, according to test data and documents obtained by Environmental Working Group (EWG). Test results never before made public show that leafy vegetables grown with contaminated irrigation water take up, store and concentrate potentially harmful levels of perchlorate, a thyroid toxin that is the explosive main ingredient of rocket and missile fuel.
Sworn depositions and other courtroom documents show that the giant aerospace and defense contractor Lockheed Martin – a major user of perchlorate responsible for widespread contamination of Southern California water supplies – knew as early as 1997 that vegetables stored high concentrations of the chemical, but said nothing to the EPA or state health officials. Since most perchlorate-related work by defense contractors is done for the U.S. military, the Department of Defense may also have known, but said nothing to warn other agencies, consumers – or farmers whose crops, through no fault of their own, may be tainted by contaminated irrigation water.
For the entire report and supporting documents, see http://www.ewg.org/reports/rocketlettuce/
So far away we have gone from “nature.”
Janine Benyus: 9 Basic Principles of Biomimicry
Nature runs on sunlight.
Nature uses only the energy it needs.
Nature fits form to function.
Nature recycles everything.
Nature rewards cooperation.
Nature banks on diversity.
Nature demands local expertise.
Nature curbs excesses from within.
Nature taps the power of limits.
Imagine our thought process at every level of human action and human interaction applying these principles in the form of limiting the damage caused by the for-profit-at-any-costs models we have been spoon fed the past few centuries.
And I thought the Dirty Dozen was a movie with Lee Marvin starring in it!
Aldrin
Listed under Annex A
A pesticide applied to soils to kill termites, grasshoppers, corn rootworm, and other insect pests, aldrin can also kill birds, fish, and humans. In one incident, aldrin-treated rice is believed to have killed hundreds of shorebirds, waterfowl, and passerines along the Texas Gulf Coast when these birds either ate animals that had eaten the rice or ate the rice themselves. In humans, the fatal dose for an adult male is estimated to be about five grams. Humans are mostly exposed to aldrin through dairy products and animal meats. Studies in India indicate that the average daily intake of aldrin and its byproduct dieldrin is about 19 micrograms per person.
Chlordane
Listed under Annex A
Used extensively to control termites and as a broad-spectrum insecticide on a range of agricultural crops, chlordane remains in the soil for a long time and has a reported half-life of one year. The lethal effects of chlordane on fish and birds vary according to the species, but tests have shown that it can kill mallard ducks, bobwhite quail, and pink shrimp. Chlordane may affect the human immune system and is classified as a possible human carcinogen. It is believed that human exposure occurs mainly through the air, and chlordane has been detected in the indoor air of residences in the US and Japan.
DDT
Listed under Annex B with acceptable purpose for disease vector control
DDT was widely used during World War II to protect soldiers and civilians from malaria, typhus, and other diseases spread by insects. After the war, DDT continued to be used to control disease, and it was sprayed on a variety of agricultural crops, especially cotton. DDT continues to be applied against mosquitoes in several countries to control malaria. Its stability, its persistence (as much as 50% can remain in the soil 10-15 years after application), and its widespread use have meant that DDT residues can be found everywhere; residual DDT has even been detected in the Arctic.
Perhaps the best known toxic effect of DDT is egg-shell thinning among birds, especially birds of prey. Its impact on bird populations led to bans in many countries during the 1970s. Although its use had been banned in many countries, it has been detected in food from all over the world. Although residues in domestic animals have declined steadily over the last two decades, food-borne DDT remains the greatest source of exposure for the general population. The short-term acute effects of DDT on humans are limited, but long-term exposures have been associated with chronic health effects. DDT has been detected in breast milk, raising serious concerns about infant health.
DDT web section covers overview, guidance, evaluation of continued use, DDT register, DDT toolkit, etc. Global Alliance for promoting a global partnership on the development and deployment of alternative products, methods, and strategies to DDT for disease vector control is also available.
Dieldrin
Listed under Annex A
Used principally to control termites and textile pests, dieldrin has also been used to control insect-borne diseases and insects living in agricultural soils. Its half-life in soil is approximately five years. The pesticide aldrin rapidly converts to dieldrin, so concentrations of dieldrin in the environment are higher than dieldrin use alone would indicate. Dieldrin is highly toxic to fish and other aquatic animals, particularly frogs, whose embryos can develop spinal deformities after exposure to low levels. Dieldrin residues have been found in air, water, soil, fish, birds, and mammals, including humans. Food represents the primary source of exposure to the general population. For example, dieldrin was the second most common pesticide detected in a US survey of pasteurized milk.
Endrin
Listed under Annex A
This insecticide is sprayed on the leaves of crops such as cotton and grains. It is also used to control rodents such as mice and voles. Animals can metabolize endrin, so it does not accumulate in their fatty tissue to the extent that structurally similar chemicals do. It has a long half-life, however, persisting in the soil for up to 12 years. In addition, endrin is highly toxic to fish. When exposed to high levels of endrin in the water, sheepshead minnows hatched early and died by the ninth day of their exposure. The primary route of exposure for the general human population is through food, although current dietary intake estimates are below the limits deemed safe by world health authorities.
Heptachlor
Listed under Annex A
Primarily used to kill soil insects and termites, heptachlor has also been used more widely to kill cotton insects, grasshoppers, other crop pests, and malaria-carrying mosquitoes. It is believed to be responsible for the decline of several wild bird populations, including Canadian Geese and American Kestrels in the Columbia River basin in the US. The geese died after eating seeds treated with levels of heptachlor lower than the usage levels recommended by the manufacturer, indicating that even responsible use of heptachlor may kill wildlife. Laboratory tests have also shown high doses of heptachlor to be fatal to mink, rats, and rabbits, with lower doses causing adverse behavioral changes and reduced reproductive success.
Heptachlor is classified as a possible human carcinogen. Food is the major source of exposure for humans, and residues have been detected in the blood of cattle from the US and from Australia.
Hexachlorobenzene (HCB)
Listed under Annex A and Annex C
First introduced in 1945 to treat seeds, HCB kills fungi that affect food crops. It was widely used to control wheat bunt. It is also a byproduct of the manufacture of certain industrial chemicals and exists as an impurity in several pesticide formulations.
When people in eastern Turkey ate HCB-treated seed grain between 1954 and 1959, they developed a variety of symptoms, including photosensitive skin lesions, colic, and debilitation; several thousand developed a metabolic disorder called porphyria turcica, and 14% died. Mothers also passed HCB to their infants through the placenta and through breast milk. In high doses, HCB is lethal to some animals and, at lower levels, adversely affects their reproductive success. HCB has been found in food of all types. A study of Spanish meat found HCB present in all samples. In India, the estimated average daily intake of HCB is 0.13 micrograms per kilogram of body weight.
Mirex
Listed under Annex A
This insecticide is used mainly to combat fire ants, and it has been used against other types of ants and termites. It has also been used as a fire retardant in plastics, rubber, and electrical goods.
Direct exposure to mirex does not appear to cause injury to humans, but studies on laboratory animals have caused it to be classified as a possible human carcinogen. In studies mirex proved toxic to several plant species and to fish and crustaceans. It is considered to be one of the most stable and persistent pesticides, with a half life of up to 10 years. The main route of human exposure to mirex is through food, particularly meat, fish, and wild game.
Toxaphene
Listed under Annex A
This insecticide is used on cotton, cereal grains, fruits, nuts, and vegetables. It has also been used to control ticks and mites in livestock. Toxaphene was the most widely used pesticide in the US in 1975. Up to 50% of a toxaphene release can persist in the soil for up to 12 years.
For humans, the most likely source of toxaphene exposure is food. While the toxicity to humans of direct exposure is not high, toxaphene has been listed as a possible human carcinogen due to its effects on laboratory animals. It is highly toxic to fish; brook trout exposed to toxaphene for 90 days experienced a 46% reduction in weight and reduced egg viability, and long-term exposure to levels of 0.5 micrograms per liter of water reduced egg viability to zero.
Polychlorinated biphenyls (PCB)
Listed under Annex A with specific exemptions and under Annex C
These compounds are used in industry as heat exchange fluids, in electric transformers and capacitors, and as additives in paint, carbonless copy paper, and plastics. Of the 209 different types of PCBs, 13 exhibit a dioxin-like toxicity. Their persistence in the environment corresponds to the degree of chlorination, and half-lives can vary from 10 days to one-and-a-half years.
PCBs are toxic to fish, killing them at higher doses and causing spawning failures at lower doses. Research also links PCBs to reproductive failure and suppression of the immune system in various wild animals, such as seals and mink.
Large numbers of people have been exposed to PCBs through food contamination. Consumption of PCB-contaminated rice oil in Japan in 1968 and in Taiwan in 1979 caused pigmentation of nails and mucous membranes and swelling of the eyelids, along with fatigue, nausea, and vomiting. Due to the persistence of PCBs in their mothers’ bodies, children born up to seven years after the Taiwan incident showed developmental delays and behavioral problems. Similarly, children of mothers who ate large amounts of contaminated fish from Lake Michigan showed poorer short-term memory function. PCBs also suppress the human immune system and are listed as probable human carcinogens.
PCBs web section covers overview, decisions, guidance, meetings, workshops, and webinars additional resources. PCB Elimination Network for promoting and encouraging the environmentally sound management of PCB with a view to attaining the 2025 and 2028 goals of the Stockholm Convention with respect to PCB is also available.
Polychlorinated dibenzo-p-dioxins (PCDD)
Listed under Annex C
These chemicals are produced unintentionally due to incomplete combustion, as well during the manufacture of pesticides and other chlorinated substances. They are emitted mostly from the burning of hospital waste, municipal waste, and hazardous waste, and also from automobile emissions, peat, coal, and wood. There are 75 different dioxins, of which seven are considered to be of concern. One type of dioxin was found to be present in the soil 10 – 12 years after the first exposure.
Dioxins have been associated with a number of adverse effects in humans, including immune and enzyme disorders and chloracne, and they are classified as possible human carcinogens. Laboratory animals given dioxins suffered a variety of effects, including an increase in birth defects and stillbirths. Fish exposed to these substances died shortly after the exposure ended. Food (particularly from animals) is the major source of exposure for humans.
Polychlorinated dibenzofurans (PCDF)
Listed under Annex C
These compounds are produced unintentionally from many of the same processes that produce dioxins, and also during the production of PCBs. They have been detected in emissions from waste incinerators and automobiles. Furans are structurally similar to dioxins and share many of their toxic effects. There are 135 different types, and their toxicity varies. Furans persist in the environment for long periods, and are classified as possible human carcinogens. Food, particularly animal products, is the major source of exposure for humans. Furans have also been detected in breast-fed infants.
http://chm.pops.int/TheConvention/ThePOPs/The12InitialPOPs/tabid/296/Default.aspx