Women who suffer with endometriosis do not have many options for treatment. As a result, many women try Lupron because they are desperate to be pain and symptom free. I was one of these women. In 2011 and 2013, I tried Lupron for a total of three doses and wish I knew then what I know now.
In 2010, I was diagnosed with endometriosis laparoscopically. After that, I tried everything from many different types of birth controls and pain medications to depression medications that can also be used to treat pain. Nothing worked. My doctor at the time suggested Lupron to me and I was desperate. I felt like I had already put my life on hold long enough because of this disease and just wanted to be out of pain. However, now I feel like I am suffering the consequences and it didn’t even get rid of my pain.
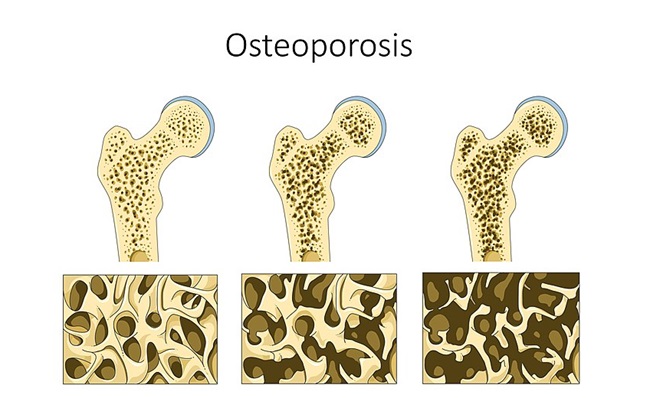
In 2015, I was diagnosed with osteoporosis. Prior to this, I had never had a bone scan done, not even before being administered Lupron. In 2009, I was diagnosed with Vitamin D deficiency and have been taking a supplement ever since. Three months before being diagnosed with osteoporosis, I stress fractured my knee and was told at my age, it should not have happened. In 2014, I had a hysterectomy because of endometriosis; I couldn’t take the pain anymore and had disease on both ovaries.
Before this, I had never had bone problems or been told that I could. I blame Lupron and strongly believe using it as a treatment for endometriosis led to me having osteoporosis. At 27 years old, I am still trying to work with doctors to determine if there are any treatments I can do because people my age having osteoporosis is rare. Many medications women use for osteoporosis could negatively impact my bones even more because of my age. If I don’t use any treatment, I could suffer from even more fractures or bone breaks the older I get. Right now, my average T-score for my left hip is -3.6 and was -3.3 when I was first diagnosed. I have no idea when my bones became so brittle. In my case, I wish I would have never tried Lupron as now I know this is one of the many side effects of this terrible treatment for endometriosis and something I will have to deal with for the rest of my life.
There are not studies done on medications for osteoporosis in my age group because there are not enough people with the disease to study. The medications my current doctor wants me to try would be a daily injection I would give myself in the abdomen for two years. They are known to possibly cause osteosarcoma, a bone cancer. Based on my history, I don’t like my odds. At this time, I don’t know how I will try to treat osteoporosis. I am planning on looking into natural ways of treating the disease and see how that goes.
As a result from my knee injury two years ago, I had to have an arthroscopic surgery. My doctor repaired my torn meniscus and removed scar tissue. It is taking me longer to heal than I anticipated and I wonder if it is because I have osteoporosis.
If doctors use Lupron for patients, they should be required to give these patients bone scans before their first dose and do follow ups yearly. It is a known fact that Lupron should not be administered in more than 12 doses over a patient’s LIFETIME. I wonder why this is?
I hope my story helps someone make a decision that is best for their body and raise awareness about Lupron. I am not a doctor, nor do I claim to be, but I am a patient that continues to live with the outcomes of having endometriosis.
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
Share Your Story
If you have experience with Lupron or other GnRH altering drugs, please share your story.
Image credit: Laboratoires Servier, CC BY-SA 3.0, via Wikimedia Commons.
This article was published originally on December 13, 2017.