In Thiamine Deficiency in Modern Medical Practice and Threats to Thiamine Sufficiency in the 21st Century, I introduced the concept that thiamine deficiency underlies many common conditions plaguing modern healthcare and identified exposures and mechanisms threatening thiamine stability. In this document, I will tackle the pattern of metabolic changes associated with the modern dietary practices leading to thiamine insufficiency, and resulting in, and sustaining hyperglycemia.
Hyperglycemia Through a Different Lens
Hyperglycemia, and the metabolic dysfunction it initiates, is a worldwide problem that has reached epidemic proportions. Due in part to overconsumption of sugary foods and in part to decrements in mitochondrial capacity that drive cravings for sugars, hyperglycemia fuels the metabolic derangements underlying obesity, type 2 diabetes, cardiovascular disease, and more recently, research suggests Alzheimer’s disease as well. These interconnected disease processes represent the top leading contributors to morbidity and mortality.
Conventional wisdom attributes these disease processes to over-nutrition and the solutions that follow involve the restriction of calories and/or the medical manipulation of the pathways initiated by hyperglycemia. Admittedly, excess caloric intake is a component, but this nomenclature suggests an overly simplified concept of nutrition; one where all that matters is calories consumed relative to calories burned. This view obfuscates the role of micronutrients in the conversion of these calories/foods into adenosine triphosphate (ATP), the energy source for all cells. It ignores the fact that the aberrant cascades so commonly associated with hyperglycemia, are merely adaptive responses to the lack of micronutrient availability and consequent reduction in ATP. Finally, through this lens, the entirety of the blame for overeating is placed upon the individual.
In reality, while the initial choices that precipitated the hyperglycemia may have been the individual’s responsibility, once these patterns become entrenched molecularly, the resulting decline in ATP drives the cravings for high-calorie foods to compensate. In a very real way, these patients are starving despite sufficient or even excessive caloric intake. It is high-calorie malnutrition, but malnutrition nevertheless. Viewed from perspective, hyperglycemia is not a disease of excess, per se, but rather, one of deficiency. As such, the opportunities for treatment are expanded beyond the typical trend to reduce, block, or otherwise override a particular pathway, and shifted towards a rebalancing of metabolic health. Here, the question is not so much which pathways should be blocked to stave off the associated deleterious effects of hyperglycemia, but rather, what does the patient need to more effectively metabolize foods into energy? What is missing from his/her diet that will reduce the body’s drive for sugars as its primary energy source? In other words, what does he or she need to be healthy?
To answer those questions, one has to look more closely towards bioenergetics and ask what micronutrients are needed to convert consumed foods into ATP and whether or not the patient’s diet provides those nutrients. Research suggests that the energy metabolism enzymes from the cytosol through the mitochondria require at least 22 micronutrients to utilize the macronutrients from consumed foods to produce ATP. Many of these micronutrients are in short supply with high carbohydrate diets (see Threats for details). Thiamine is top among them, and because of its gateway role in energy metabolism, thiamine insufficiency is a significant contributor to the disease processes currently attributed to hyperglycemia.
Thiamine, Sugar, and Energy Metabolism
Thiamine is a required and rate-limiting co-factor to five enzymes involved in energy metabolism, including those at the entry points for the glucose, fatty acid, and amino acid pathways (transketolase, pyruvate dehydrogenase complex [PDH], 2-Hydroxyacyl-CoA lyase [HACL], and branched-chain alpha-keto acid dehydrogenase [BCKAD] and alpha ketoglutarate dehydrogenase [a-KDGH]. Insufficient thiamine leads to poor glucose handling resulting in hyperglycemia. It also induces poor protein and fatty acid metabolism resulting in the elevated branch-chain amino acids and dyslipidemias common to patients with hyperglycemic metabolic syndrome.
Conversely, high carbohydrate diets increase the demand for thiamine, which, if left unchecked, ultimately leads to thiamine deficiency, hyperglycemia, disturbed protein, and fatty acid metabolism. In healthy, thiamine-sufficient adults, high carbohydrate consumption results in a significant reduction of mean plasma thiamine concentrations in just over three weeks. Over the longer term, a high carbohydrate diet initiates many changes in thiamine and energy metabolism that ultimately result in reduced thiamine availability, higher circulating glucose, and poor energy metabolism. Thus, whether by cause or consequence, low thiamine and hyperglycemia are inextricably intertwined. One eventually leads to the other.
Altered Metabolism and Mechanisms of Damage
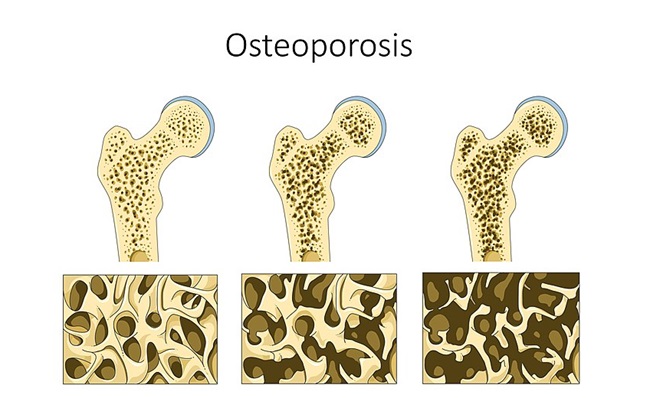
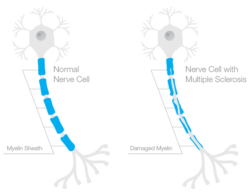
Under normal glycemic conditions and where thiamine is sufficient, excess sugars from glycolysis are shuttled through the pentose phosphate pathway via the thiamine-dependent enzymes transketolase to PDH and onward through the mitochondria. Under conditions of high carbohydrate intake/low thiamine, however, these sugars are diverted away from the primary metabolic pathways used for ATP production, inducing a net decline in ATP, and away from the synthesis of ribonucleotides and NADPH, substrates for RNA/DNA, and fatty acid metabolism and ROS detoxification respectively, to secondary metabolic pathways, specifically, the polyol/sorbitol, hexosamine, diacylglycerol/PKC, advanced glycation end product (AGE) pathways. Research suggests the upregulation of these pathways underlie the macro-and microvascular cell damage attributed to hyperglycemia, related cardiovascular and neural damage, while the decrements in ATP drive the general metabolic dysfunction associated with obesity and a host of other inflammatory conditions.
The high carbohydrate/low thiamine diet disturbs amino acid and fatty acid metabolism as well. Elevated branched-chain amino acids (BCAA) are common with hyperglycemia. Indeed, elevated BCAA may predict impending diabetes. Underlying the elevated BCCA is impaired catabolism due to a genetic or environmentally triggered defect in the BCKAD enzyme. BCKAD is dependent upon thiamine and elevated BCCAs are a manifestation of deranged energy metabolism precipitated by thiamine insufficiency. Genetic aberrations of BKCAD display similarly elevated BCAA, though typically much earlier, and respond favorably to thiamine supplementation.
With chronic hyperglycemia, the increased branched-chain keto acids, a secondary effect of poor BCAA catabolism, lead to excess short and medium-chain acylcarnitines. Surplus acylcarnitines increase the flux of fatty acids through the b-oxidation pathway beyond its capacity. This results in incomplete fatty acid metabolism, the dyslipidemias noted with hyperglycemia, and the formation of the pro-inflammatory diacylglycerol and ceramides that reinforce insulin resistance.
All of this, of course, comes against the backdrop of declining ATP capacity. Under conditions of insufficient thiamine/hyperglycemia, ATP production may be reduced up to 70% depending upon the severity and chronicity of disordered metabolism, the organ or tissue in question, and the model used to test. Decrements in the brain and heart, because of their high energy demands are the most severe, while reductions in the GI system and musculature present most noticeably in the early stages. Fatigue, weakness, and GI disturbances are among the earliest and most common unrecognized symptoms of the initial stages of insufficient thiamine.
Correcting Metabolic Dysfunction With Micronutrients
Ideally, ill-health would precipitate dietary changes, but in the case of hyperglycemia, particularly when it is chronic, the altered metabolic pathways and reduced capacity to synthesize ATP from consumed foods make this prospect difficult to impossible for some. Based upon thiamine’s role in this process, a more amenable approach might be to address thiamine and other micronutrient deficiencies first. Research from multiple disciplines demonstrates the remarkable improvement in metabolic capacity with thiamine repletion suggesting that simply replenishing this and other micronutrients may slow or reverse the progression of disease in these populations. Below are a few of the hundreds of studies published on this topic.
- Thiamine reduced or reversed hyperglycemia-related activation of the secondary glucose pathways (polyol/sorbitol, hexosamine, diacylglycerol/PKC, AGE) via upregulation of the PDH enzyme. It improved cardiac contractility, reduced cardiac fibrosis and decreased the expression of the mRNA-associated proteins (thrombospondin, fibronectins, plasminogen activator inhibitor 1, and connective tissue growth factor), and prevented obesity in the overfed arm of an experiment using streptozotocin-induced diabetes in rats.
- In streptozotocin (STZ)-induced diabetic rats, high-dose thiamine and benfotiamine (a synthetic S-acyl derivative of thiamine) therapy increased transketolase and PDH activity increasing ribose-5-phosphate and reduced microalbuminuria and proteinuria by 70-80%. PKC, AGE, and oxidative stress were all reduced significantly.
- In STZ-induced diabetic/leptin mutant type rats, benfotiamine improved heart function and prevented hyperglycemia-induced, left ventricular end-diastolic pressure increase and chamber dilatation in both models.
- Benfotiamine administration 150mg thiamine daily thiamine significantly reduced blood glucose within a month, in a randomized, placebo-control trial of 24 drug naïve T2D diabetics.
- In a three-month randomized placebo controlled trial, 50 T2D patients in the experimental arm were given 3X 100mg thiamine per day. Thiamine therapy significantly improved microalbuminuria, glycated hemoglobin, while decreasing PCK levels. Markers of oxidative stress and fibrinolysis were non-significant.
- After 45 days of benfotiamine and vitamin B6 supplementation, 19 of the 22 patients enrolled in the study saw statically significant reductions in pain, symptom scores, neurophysiological and biological markers of diabetic neuropathy.
- A 6 month randomized trial with 60 T2D with medication-controlled blood sugar and 26 age – and BMI-matched controls found that 100mg thiamine daily, significantly corrected lipid profiles and creatinine levels.
- One time administration of 100mg IV thiamine, improved endothelium-dependent vasodilatation in 10 patients with TD2 during an acute glucose tolerance test.
- One week of IV thiamine administration at 200mg/day in six patients with heart failure (HF) and who were also receiving diuretics (diuretics deplete thiamine) improved left ventricular ejection fraction (LVEF) in four of those patients from 24% to 37%.
- A randomized, double-blind, placebo controlled study of HF patients on diuretic treatment found that 300mg/day oral thiamine improved LVEF significantly.
Thiamine Insufficiency Versus Deficiency
Among the more common misperceptions about thiamine is that deficiency is delineated by laboratory testing. While this is true for severe deficiency and when the appropriate laboratory tests are utilized, far too often, the insufficiency syndromes that present months to decades before frank deficiency is detected, are missed completely. This owes in part to the variability of testing methodologies and in part to the very framework from which we determine sufficiency and deficiency. Thiamine testing, like the tests for many micronutrients, carries a high false-negative rate and fails to consider the nature of micronutrient deficiency relative to need. The next paper in this series will addressing testing methods.
As outlined above and in the Threats document, several environmental variables increase the demand for nutrients, a diet high in carbohydrates is top among them. The increased demand will not necessarily or immediately test positive for deficiency. Rather, it will present symptomatically and must be suspected based upon the symptoms of deranged energy metabolism. In these cases, thiamine supplementation is done to support and correct reduced enzyme activity so that consumed foods may be more efficiently metabolized and converted into ATP. This then reduces the use of the less efficient and generally deleterious secondary metabolic cascades linked to the constellation of negative health effects associated with hyperglycemia.
Consider Thiamine
Thiamine is a safe, non-toxic, essential nutrient that has become increasingly difficult to maintain in the face of modern dietary practices and chemical exposures. Thiamine sufficiency is fundamental to energy metabolism, mitochondrial capacity, and thus, health. Consider thiamine in your practice.
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
Image created using Canva AI.











Dear Mrs. Marrs,
First of all, thank you for your work and the work of Mr. Lonsdale. since March I have had a horror story. it seems I had a more severe thiamine deficiency. I have had several strange symptoms, including insomnia, tingling and burning in my hands and feet, feeling almost like dying, too weak to eat, trembling, irritability, high pulse. The tingling started a few weeks earlier, but I thought it was a magnesium deficiency and took magnesium. What had helped sometimes.
My naturopath advised me to take benfothiamine. It helped me a lot. One morning I also had an almost complete loss of energy in my body. I thought I was really going to break down and collapse. My body felt like it was flickering, probably due to energy loss (mitochondria?). Only benfothiamine in combination with food gave me back my energy. In the beginning I had to take a 150mg Benfothiamine every 5-8 hours, otherwise I felt like I was breaking down and food was no longer giving me energy in my body.
There was no fuel left in my brain or CNS. I also had neurological problems, pulling in the back of my brain and burning at times. Fortunately, an MRI of my head showed no problems. Walking normal distances was no longer possible, my CNS felt powerless. I have read a lot about thiamine deficiency. I don’t know if I got beriberi. I actually live quite a healthy life, don’t drink alcohol and don’t smoke. I have my blood values checked every year. Had had covid 2-3 times. And also post Covid problems.
Last year a doctor gave me the tip to live gluten-free. However, I only ate gluten-free bread but wasn’t completely gluten-free. I think maybe that’s what stole my thiamine. At the beginning of March I also had some kind of virus, it was very strange. At the moment I still take B-complex, magnesium 250mg and 150-300mg benfothiaine per day. What’s really strange is that I have a metallic taste in my mouth, which usually comes from my upper jaw. Sometimes better, sometimes worse. When I started taking Benfothiamine it was very extreme. I thought it was some kind of detox and thought I was poisoned and it was stealing my thiamine.
I have been to many doctors.
No doctor here could really help me. Many of them didn’t really believe me. Many neurological tests were carried out and everything was ok. It also seems that my pancreas may have a problem. This is still being investigated further. I did some blood and urine tests for toxicity, minerals and vitamins. The results didn’t show any toxicity.
Long story short: do you have any ideas about the metallic taste in connection with thiamine? When I have a lot of metallic taste I feel worse and get nauseous, shaky, etc. Fortunately, this has all gotten much better, thanks to thiamine. Thank you in advance. Ben
I am not sure the metallic taste is relative to thiamine, except that thiamine upregulates the use of other nutrients and can create/unmask latent deficiencies. To that end, some possibilities: low b12, zinc, iron, both high and low copper, and high selenium. It could also be an infection or some exposure to mold or other toxins. I am not sure gluten has anything to do with it, but in general grains, especially non-organic ones are problematic for other reasons. Have you had other nutrient tests done, like an OAT test?
Hello, thank you for your feedback. I really appreciate this. Well, the last blood test from 2 weeks ago hasn’t shown a real mineral/vitamin deficiency. B12 is 648pg/ml (is in normal Range, but could be better), b1 (bioactive) & b3 was too high, b6 & b9 normal, zinc, iron and ferritin is ok, selenium is a bit down but still in normal range and copper is on the low normal edge (but copper was always a bit low since I had the first covid). Vitamin D is gone down (33ng/ml). Yes, an infection or toxin/mold is a possibility what I have also thought. I am also not sure if an autoimmun disease could do such a thing. I already changed a few things in my environment but have no real clue. Toxic Metall tests in blood and urine was negative. Last year I had a big microbiom test and a nutrition (immun blood) test done.
OAT is organic acid test ? I am from Germany and I have found a lab who could do this (a bit expensive) but seems worth to me. Also I will go again to my dentist to double check everything.
All in all I can say thiamine helped me massive to come out of this horrible situation and almost all symptoms I had points to a thiamine deficiency but the cause is unclear for me. However, the feeling was really horrible it felt for me as my cns had a total oil change. Not good at all. In the last days it looked for me that I can reduce now the thiamine intake but I will sensitive listen to my body.
I will keep you updated. Thanks again. Ben