Since the early 20th century, calories have been equated to health and energy. The assumption is that calories, no matter their composition, equal energy, and energy equals health. Strictly speaking, this is correct. The body will take whatever it is given, and to the best of its ability, convert it into cellular energy or adenosine triphosphate (ATP). That process produces heat and heat is the unit of change underlying the concept of calories. Mathematically, a calorie or kilocalorie (kcal), the unit used in food science, represents the heat or energy required to raise the temperature of 1 kilogram (kg) of pure water to 1° Celsius. This equation is used to calculate the heat content of food and determine the amount of heat energy produced as the food passes through the body. Another simple metric called the basal metabolic (BMR) is used to calculate the energy one requires to survive. From these two metrics, we get the calories in/calories out framework that dominates conversations about health and disease.
According to this framework, if the two numbers balance, we should have sufficient energy to meet the demands of daily living. If we consume more calories than we burn then, we should have excess energy, which can be used to increase one’s activity level or be stored as potential energy for use in the future. In today’s sedentary environment, it is usually the latter. Conversely, if we consume fewer calories than necessary, decrements in energy and weight loss should follow. In either case, energy is simply a matter of physics. Heat energy is transferred from one source – the food, to another – the body, or from the body to the environment at large, as one uses the energy in daily living and it dissipates.
This is the framework that has guided the medical profession, the food industry, and countless weight loss gurus for generations. To that end, it is of little to no concern what these calories are comprised of and very little thought is given to the endogenous processes underlying the generation of heat or energy. This framework allows us to represent health and illness mathematically. It is simple, recognizable, and easily understood, and perhaps that is why we hold dearly to it but is it accurate? What if there is more to this story? What if energy is not just a matter of heat transfer and what if the content of the calorie matters as much or more than the calorie itself? To answer that question, we need to determine what type of energy the body needs to survive and how that energy is derived.
What is Energy?
If energy is not a matter of calories, at least not in the sense that is portrayed, what is it then? In the body, energy comes in the form of ATP molecules, produced primarily by the mitochondria. In broad terms, ATP is the result of a series of reactions that combine oxygen with components of metabolized foods. Part of this process takes place in the cell but most of it takes place in the cell’s power plants, the mitochondria.
If all is going well, derivatives of fat, protein, and carbohydrates, the macronutrients contained in food, are shuttled into the mitochondria, through what is called the tricarboxylic acid (TCA) cycle (also called the Krebs or citric acid cycles), combined with oxygen, and through a series of reactions, collectively called oxidative phosphorylation (OXPHOS), produce ATP. From the oxidation of one glucose molecule, we get around ~30-36 ATP molecules. From one fatty acid molecule, we get over 100 ATP molecules, and from the amino acids, we get either substrates for the synthesis of other proteins, more glucose to metabolize and feed into the mitochondria (amino acids can be converted into glucose via gluconeogenesis), or another compound called pyruvate that may also be used by the mitochondria to make fuel or converted into lactate.
When things are not going as well or when quick energy is needed for certain functions, a number of extra-mitochondrial pathways, are used to break down the various macronutrient components to provide energy. There are two key pathways here. One is called glycolysis and the other is called the pentose phosphate pathway (PPP), which connects glycolysis with the TCA and OXPHOS and performs a few other important tasks like providing substrates for DNA and RNA synthesis. The PPP nets 1 ATP molecule per molecule of glucose, while glycolysis nets about 2 ATP molecules per molecule of glucose. A third pathway, used primarily in quickly replicating immune cells and cancer cells, involves shuttling glutamine, an amino acid, through the side door of the mitochondria to produce ~24 units of ATP per unit of glutamine. Severely stressed neurons use this pathway as well.
Which pathway is used to produce ATP may alternate according to to need, cell type, fuel type, micronutrient, and/or oxygen availability. Some cells metabolize a good portion of their energy in the cytosol using glycolysis while others rely almost exclusively on the OXPHOS in the mitochondria. This flexibility allows cells to adapt rapidly to changing circumstances. If one macronutrient is not available, energy is derived from another. When a micronutrient (vitamin or mineral) is not available, the products are diverted through other pathways, and when there is limited oxygen, glycolytic pathways will take over. All of this is meant to help the body metabolize different compounds and maintain energy homeostasis relative to environmental demands.
Different Energy Pathways for Different Cells
The body requires an enormous amount of energy to meet the demands of life. We effectively turn over our weight in ATP every single day. Every. Single. Day. That is absolutely remarkable. When we exercise, we produce even more – 0.5 to 1.0 kg per minute. Fully 95% of this energy comes from mitochondria, making mitochondrial fitness of the utmost importance. Many cells contain anywhere from 1000 to 2500 mitochondria and can represent from 25% to upwards of 60% of the cell volume. Accordingly, the average cell may use upwards of 10 billion units of ATP per day.
The production of ATP is critically important for survival and so its manufacturing of ATP is inherently dynamic and adaptable. The body will take whatever it is fed and turn it into energy using whichever pathway is available. So even when we feed ourselves garbage food, the body will do its best to manufacture some quantity of energy. The body does, however, have preferences. Importantly, different types of cells favor specific fuel types or pathways.
Skeletal muscles, for example, use fatty acids shuttled through the mitochondria for fuel when at rest but switch over to glucose, creatine, or lactate via glycolysis as exercise intensity and/or duration increase. Creatine, synthesized in the liver from glycine (and taken as a popular supplement in the athletic community), is part of a system that effectively recycles ATP in skeletal muscle during intense bouts of exercise. Lactate, originally believed to be a waste product, is actually an important fuel source. The ability to repurpose lactate, and metabolize it into ATP, whether via glycolytic pathways or via derivatives that will enter the mitochondria, is a major determining factor in forestalling fatigue for athletes and non-athletes alike.
You might be thinking, if glycolysis produces less energy per unit of glucose, why would the body choose to use it? Two reasons. The first is speed. Sometimes the energy is needed more quickly than can be provided by the mitochondria. So the body trades some capacity for speed. This occurs in fast-growing cells like the immune cells that need to replicate quickly during an infection. Similarly, high-energy situations like intense and quick bouts of exertion favor glycolysis. These are normal and adaptive. Secondly, glycolysis and its sister pathway, the PPP, also provide important components for cell building. If that is what is required, those are the pathways that will be used.
Unfortunately, glycolysis is also favored when the mitochondria are struggling. Here, molecules that would normally be shuttled into the mitochondria are diverted into other paths, and energy production is diminished. This is common in patients with metabolic disorders. The excess intake of sugars often paired with a lack of micronutrients overwhelms mitochondrial capacity, and as a result, much of the glucose remains in the cell and is either metabolized into what few ATP the cell can muster via glycolysis or shuttled towards other pathways that will act as waste management and expend rather than create energy. Cancer follows this pattern.
The continuously active heart cells require a huge amount of energy, approximately 6 kg of ATP per day to pump blood, most of which comes from the mitochondria. At rest, ~90% of the heart’s ATP comes from the oxidation of fatty acids in the mitochondria. During exertion, there is a slight shift in substrate preference and more glucose is used. Instead of a 90/10 split of fats to glucose, with activity, the split is closer to 60/40. In either case, these numbers show us that mitochondrial capacity and diet are incredibly important for heart health. Low fat, high carbohydrate diets, as have been recommended for decades, go against the fuel preference of a healthy heart. The result of this type of diet is evident in the cardiovascular disease associated with metabolic syndrome. With metabolic syndrome, which represents nothing more than dietary damage accrued over time, the mitochondria lose the capacity to metabolize fatty acids for ATP and instead must rely exclusively on glucose for energy. The resulting decrements in energy underlie many of the aberrant patterns in rate, rhythm, and pressure. The heart simply does not have the energy to pump effectively at rest but especially under stress. Importantly, with metabolic syndrome, dysfunction expands beyond the heart, and other cells will also lose the capacity to metabolize fatty acids, gradually shifting to a more glucose/glycolysis dominant metabolism.
The brain consumes a substantial amount of glucose to meet energy needs (5.6mg of glucose per 100g of brain tissue per minute), with neurons using up to 80% of that energy. While the brain represents only about 2% of the body’s mass, it consumes 20% of the daily energy budget. Since the brain and the nervous system effectively manage all aspects of survival, decrements in energy metabolism have deleterious effects not only on the range of behaviors typically attributed to the brain, thinking, memory, planning, speech, emotion, movement, and the like but also on the automatic or autonomic control of organ function. For example, the parts of the brain located in the back of the head, collectively called the autonomic system (the cerebellum and brainstem, together with the nerves that flow through the spinal cord to the various organs and tissues), are exquisitely sensitive to changes in energy availability. The brainstem especially, because it controls breathing and heart rate, the two most important functions for survival, requires massive quantities of ATP. When energy availability is compromised, heart rate, breathing, and other autonomically controlled systems become dysregulated leading to what is now called dysautonomia.
Although most of the brain’s energy is derived from the oxidative phosphorylation of glucose within the mitochondria, here too, lactate recycling, extra-mitochondrial pathways like the PPP and glycolysis also play a role. Additionally, as ketogenic diets have shown us, the brain may use ketones derived from fatty acids as a fuel source.
Even proteins may be used for brain fuel. This is a relatively new discovery and not widely appreciated, but a set of neurons in the hypothalamus called the orexin or hypocretin neurons (same neurons, different names), require amino acids to fire. Specifically, and in order of potency, glycine > aspartate > cysteine > alanine > serine > asparagine > proline > glutamine induce orexin firing. This is important because these neurons are the primary energy sensors in the brain. They are responsible for maintaining wakefulness, providing the motivation to eat, and monitoring brain energy levels as a whole. Mutations in these neurons are responsible for narcolepsy, but due to their energy-sensing role, any disruption in brain energy, may force sleep and induce anorexia. In other words, these neurons control survival functions that become disrupted when ill. Low concentrations of orexin/hypocretin lead to what is called ‘sickness behaviors’ – the behaviors that every organism exhibits when ill. These neurons are also involved in precipitating migraine implicating brain energy deficiency here too. Interestingly, unlike other neurons in the brain, where glucose spurs activity, in these neurons, glucose spurs inactivity, perhaps through associated inflammation. Glucose, particularly high glucose, will cause these neurons to stop firing, which may be perceived as excessive fatigue, an insatiable need for sleep, and when severe enough, coma.
Of interest, the most important amino acid for the proper activity of these neurons is glycine. Glycine is an essential amino required for protein synthesis and repair. It is also an excitatory neurotransmitter in its own right, affecting other neural systems. Glyphosate, the chemical used on virtually all commercially grown agriculture (and thus, consumed by all commercially grown livestock), is a glycine analog. That means that whenever we consume commercial foods where glyphosate-based herbicides are used liberally, we are substituting natural and endogenous glycine for a synthetic analog. This substitution has a long list of health-derailing effects. Another ill-effect to add to that list may be the inappropriate regulation and responsivity of the orexin/hypocretin neurons.
The Composition of Calories
The section above illustrates the necessity for providing a variety of whole and uncompromised foods to fuel the body and it should fundamentally shift how we perceive the energy capacity of different food types. The body requires a variety of fuel sources to function appropriately. From the calorie-focused perspective of energy, none of this matters. It is assumed that so long as there are ample calories, energy production will be maintained at sufficient levels. The makeup of those calories is inconsequential to ATP output. This is clearly incorrect. For even if we look only at the raw numbers of units of ATP per pathway, it is evident that the composition of the diet matters. Someone who eats a predominately carbohydrate-based diet will produce quantitatively fewer ATP molecules than someone whose diet derives the bulk of their calories from fats. Similarly, the ability to funnel macronutrient components through the mitochondria and to run OXPHOS will produce more energy than if one’s cells are stuck in the glycolytic pathways. When a diet is skewed towards one type of food, the pathways that rely on the other macronutrients will be impacted negatively and this, in turn, will affect the organs that prefer one type of fuel over another.
If we dig a little deeper and look at the composition of consumed carbohydrates and fats, there are even more differences to consider. For example, carbohydrates coming from refined sugars like high fructose corn syrup (HFCS) produce less ATP than those that come from whole and unadulterated grains, fruits, or vegetables. In fact, the metabolism of HFCS requires ATP rather than produces it, and as an added complication, a good portion of the metabolized products derived from HFCS never enter the mitochondria but are instead converted to triglycerides and stored as fat. Similarly, consumed fats that come from animal fats versus seed oils, differ in their ability to produce ATP. Soybean oil, an oil extracted from soybean seeds, not only incites inflammation and a host of other ailments, but it downregulates the enzyme that sits at the entry point to the mitochondria, effectively blocking glucose metabolism and shifting everything to glycolysis for a huge net loss in ATP production. Since all heavily processed foods contain both of these ingredients, consuming these products with any regularity diminishes, and likely damages mitochondrial function. The net result is poor energetic capacity.
And if we dig deeper still, we find that the thousands of chemicals used to grow, preserve, enhance, and package these products, leach nutrients, derail mitochondrial functioning, and in many instances, evoke mitochondrial cell death. Consuming these foods, as so many of us are inclined to do regularly (57% of kcal in the American diet is composed of ultra-processed foods), leads to poor mitochondrial function and limited energetic capacity. It is not just the processed foods that have become problematic though. Conventionally grown produce is less nutritious than what was grown a few decades ago before the adoption of glyphosate-based herbicides and the genetically modified plants designed to withstand these chemicals became so pervasive. As discussed previously, glyphosate-based herbicides like Roundup that are ubiquitous in conventional agriculture (1.8 billion pounds of glyphosate used annually, enough for 4 pounds per person per year), block glycine. These herbicides also chelate (remove) minerals from the soils and plants and from the humans who consume these products. Minerals like calcium, magnesium, zinc, and manganese, which, as we will see later, are critically important for mitochondrial function. Indeed, the original patents for glyphosate involved its industrial descaling capacity, exactly the mechanisms enacted in the human body. It should be noted though, that glyphosate is just one of the tens of thousands of chemical toxins we are exposed to daily, most of which have never been tested for safety but instead are assumed to be safe under the poor regulatory template called GRAS – generally recognized as safe.
Each toxin that we ingest (or breathe), requires an ATP-using response from the body, thus diminishing potential reserves by some quantity. One can imagine, how over time the repeated consumption of these types of products might fundamentally alter mitochondrial function and reduce ATP capacity. Importantly, since the gastrointestinal (GI) system provides the interface between consumed foods and the rest of the body and is responsible for the digestion, absorption, and metabolism of food-based nutrients and excretion of toxicants, reduced mitochondrial functioning e.g. reduced ATP in the GI system is doubly problematic. Not only is GI functioning disrupted and oftentimes damaged by these types of foods, but the ability to derive nutrition becomes impaired as well. It takes energy to make energy and it takes energy to extract and metabolize nutrients and excrete waste products. Commercial foods, while high in calories and non-caloric additives are low in energy. These foods lack actual nutrients and nutrients are what mitochondria need to make energy.
How Do We Fix This Mess
It should go without saying that we ought to eat better and avoid food and other toxins where we can, but the food landscape is such that this can be difficult, especially if one is already ill and reactive to many foods and/or other substances. In those cases, it is important to understand what it takes to make energy from food, determine what is potentially missing from your diet, and replenish accordingly. This is not easy and will take a fair amount of detective work on your part, but it is possible.
We briefly covered the macronutrients, here we will look into the micronutrients. Micronutrients are vitamins, minerals, and some metal ions. In generations past, before we sterilized the soils with chemicals and modified the plants to withstand those chemicals, one could consume a complement of these micronutrients so long as one had a reasonably balanced diet. It is here where the concept of calories made a little more sense when food was food and not some commercially derived concoction. That is no longer the case. The advent and escalation of herbicides, pesticides, and the slew of additives, preservatives and other noxious chemicals in the food chain have effectively stripped modern foods of nutritional capacity while retaining caloric content. This means, that for many people, supplements will be required. Which ones and what dosages, however, will vary significantly. For that reason, it is important to understand how the mitochondria work so that you may become your own expert.
Broadly, for foods to be metabolized into energy, for any macronutrients to enter the mitochondria and run OXPHOS, vitamins and minerals must be available to power the enzymes leading to and through the mitochondria. If there are insufficient vitamins and minerals both in general and relative to the concentration of macronutrients (high calorie, low nutrient foods) or demand (toxins, stressors, illness), the TCA cycle does not work, OXPHOS does not work well, the body has to shift to alternate pathways. With this shift, not only is ATP reduced but because these pathways burn dirtier, more endogenous pollutants, like reactive oxygen species (ROS), are released. The oxidative stress that ensues damages mitochondrial membranes, further taxing mitochondrial capacity. This damage simultaneously demands more energy to resolve while reducing the capacity to produce that energy. Over time, the ability to manufacture ATP becomes so disrupted and produces so much oxidative stress that the entire process of extracting and metabolizing foods into energy further damages the mitochondria. Eating the very nutrients the body needs becomes a stressor and the individual becomes stuck in a seemingly never-ending negative cycle of malnutrition causing more malnutrition with any attempts to rectify inducing negative reactions. This is the state I find many people with chronic illness in – in desperate need of nutrients but unable to consume them.
Let us look briefly at the micronutrients involved in deriving energy from proteins, carbohydrates, and fats. Below is a graphic from the book I co-authored with Dr. Derrick Lonsdale. While it focuses on thiamine, it lists many of the other nutrients required for mitochondrial metabolism. Notice that within each pathway, a variety of vitamins, minerals, and metal ions are necessary to power the multiple enzymatic reactions require to produce ATP. The B vitamins and magnesium, in particular, play an important role in the early phases of these processes.
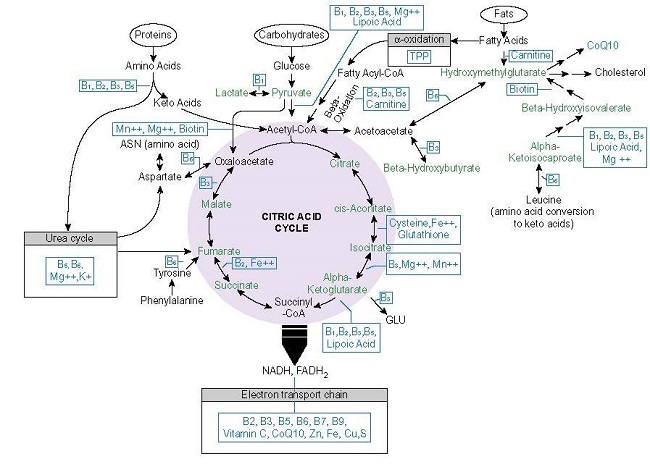
If any of those micronutrients are missing or are in short supply, the enzymes requiring those nutrients will not work as efficiently and the capacity to produce ATP will decline. With that decline comes a slew of compensatory reactions that will reallocate resources based on energy availability. Those reactions frequently involve inflammation, altered hormone regulation, and other adaptive measures, as reduced energetic capacity is a signal to other mitochondria and other cells that something is wrong.
Nothing works without energy and energy is impossible without the vitamins and minerals that drive mitochondrial function. Not even respiration is possible. Cellular respiration, the most fundamental form of respiration, the activity that breathing itself, requires critical micronutrients. Oxygen cannot be used or trafficked appropriately creating a state of hypoxia. Hypoxia, I believe, is what drives most modern illnesses. So let us take a look.
Micronutrient Deficiency Driven Hypoxia: The Root of All Illness
Among the least well-recognized reactions to reduced nutrients is a type of hypoxia called molecular or pseudo-hypoxia. Here, unlike the typical obstructive hypoxia, nothing is blocking or preventing oxygen intake. What is missing are the micronutrients required to power key enzymes involved in oxygen utilization. Specifically, for cells to breathe and to utilize oxygen to produce energy, the mitochondria require adequate thiamine (vitamin B1), magnesium, and riboflavin (vitamin B2). Looking at Figure 1, you will notice that thiamine and its activating cofactor magnesium appear frequently throughout each of the pathways used to convert foods into energy. Indeed, they are what are called rate-limiting co-factors in these processes. Meaning that if their levels dip, everything else downregulates as well.
Importantly, thiamine, magnesium, and riboflavin, along with alpha-lipoic acid, are integral to the functioning of an enzyme complex called the pyruvate dehydrogenase complex (PDC). PDC sits atop the mitochondria and acts as a gatekeeper of sorts. With insufficient concentrations of these micronutrients, the metabolism of glucose into ATP is blocked. The metabolites of other macronutrients, after some processing also utilize the PDC as an entry point, and so they too will be blocked from entering the mitochondria. As a compensatory reaction, the mitochondria initiate a series of reactions that signal danger. Among them is the release of proteins called hypoxia-inducible factors or HIFs for short. Once released, HIFs then signal all of the other changes consistent with chronic illness like inflammation, hormone reregulation, altered immune responsivity, etc. These are meant to be short-term protective measures that reduce energy requirements and increase blood flow and oxygen to the cells. Unfortunately, because these are nutrient-driven reactions, they will not be resolved until the nutrients come back on board consistently. As a result, these patterns become entrenched, and therein lies the root of many chronic illnesses.
Symptomatically, early one and when this set of reactions is limited to specific tissues, injury and inflammation will appear regional to those tissues or organs. The GI system, both because it sits at the interface between food consumption and nutrient absorption and because the microbes that inhabit the GI tract also require thiamine, will often show disruption first. The poor nutrient landscape not only impacts energetic capacity, disrupts peristalsis, and the movement of foods through the tract, but also shifts the microbial ecosystem towards more pathogenic microbes that adapt more easily to the nutrient-starved environment.
When nutrient deprivation goes on long enough and the HIFs become stabilized, not only do we see all of the compensatory reactions mentioned above, but when severe enough, we will see underlying molecular hypoxia manifest like a sensation that one cannot get enough oxygen, even though oximeter readings are perfectly normal. This is frequently referred to as air hunger.
Whatever the individual response, however, since mitochondria control life and death cycles at the cell level, ailing mitochondria that cannot manage these cascades effectively, die a messy, necrotic death that is highly inflammatory and immune reactive. What little intracellular ATP is available to power cell function is spit out of the cell and used as a danger signal to other cells. High levels of extracellular ATP are indicative of severe mitochondrial stress. When this happens, even less intracellular and intra-mitochondrial ATP is available to power basic survival functions, and importantly, to create more energy.
This begins the downward trajectory of chronic illness where one needs energy to make energy but simply does not have it; where one needs key nutrients to resolve the energy crisis but does not have the energy to metabolize those nutrients.
Resolution and Prevention
Ideally, we would prevent the downward trajectory of mitochondrial illness, but modern life, such as it is, presents innumerable threats to mitochondrial energetics. The biggest, of course, is poor diet. By focusing on the caloric content of foods, and the ease and speed at which we can prepare those foods, rather than their capacity to provide critical nutrients, we have missed the physiological purpose of eating – to provide energy to live and to function. In light of what is required to create energy from food, balanced macronutrients with an array of micronutrients, we must consider the composition of the foods we eat, especially when one is dealing with a chronic and seemingly treatment-refractory illness.
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, and like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
This article was published originally on July 19, 2022.













Dr Marrs, with low level of thiamine, would you say it is okay to take 50 mg of thiamine while taking 1mg of lorazepam every day for anxiety? Thank you!
It should be, but everyone is different in how they respond initially. Some folks can take a reasonably high dose right of the gate and respond positively, while others have to begin with single milligram dosing.
Exceptionally well-written article! Enjoyed this one.