“Meet the new boss. Same as the old boss.”
– The Who (Pete Townsend, Songwriter), 1971
The FDA recently finalized its ruling to ease informed consent requirements for “Minimal Risk Clinical Investigations.”
As you might expect, legacy media turned a blind eye. Admittedly, “easing requirements” and “minimal risk” do not readily inspire hard-hitting headlines, but this is precisely the type of ruling that should, at the very least, give us pause.
The entirety of our faith in the medical system relies on two tenets. First, we assume the precepts of informed consent provide a guaranteed layer of protection for both patients and test subjects of scientific research. Second, we assume that doctors take an oath to “first, do no harm.”
So, before we simply dismiss this new ruling as inconsequential, let’s first look at the system in which we have placed such great trust.
Is it Hippocratic or Hypocritical?
We will begin with the latter since it is easier to tackle. Most of us outside the medical industry still tend to think that the Hippocratic Oath is as much a part of becoming a doctor as spending time in a residency program. However, that is not the case, and a recent survey of over 2,800 physicians and medical students confirmed an alarming trend for those of us who may take a bit of comfort in the oath.
Among doctors 65 and older, 70% said the oath was very meaningful, while less than 40% felt that way among those physicians under the age of 35. Of the younger group, nearly one out of every five said the oath was not meaningful at all.
Does this mean the young doctors and medical students lost faith in the oath? Not necessarily. One doctor’s comment suggests he may have lost faith in the system. He wrote that the oath is “sadly, irrelevant. Medicine has evolved from a profession into a huge service industry that involves many other players. These players, like health insurance, hospital employers and pharmaceuticals, do not pray to the same god as the medical profession. Their priority is financial profit ― within or without the Hippocratic Oath.”
The Evolution of Informed Consent
The dubious state of medical oaths means our safety depends much more on informed consent.
The concept of informed consent began to evolve very slowly after the creation of the Food and Drug Administration (FDA) in the early 1900’s. At first, a smattering of landmark lawsuits began to highlight the need for greater protections for patients and test subjects. Some of the lawsuits revolved around doctors going rogue, while others delved into the intricacies of health illiteracy and a patient’s need for more complete information delivered in layman’s terms.
As so often happens within bureaucracy, the FDA recognized the need for better regulations, but potential solutions floated around nebulously in the ether until things reached a breaking point on the global stage.
A Serious Catalyst
Nothing could have demonstrated the need for informed consent in medical research more than the atrocities committed by Nazi doctors in World War II. The subsequent Nuremberg Trials put the world on high alert. Across the globe, citizens were horrified to witness the depths of depravity that human souls could engage.
At the conclusion of the trials in 1947, the judges issued what is now known as the Nuremberg Code, a document outlining ten essential points necessary for any medical trial to be permissible. The first principle of that code states, “The voluntary consent of the human subject is absolutely essential.”
The authors added further context to reinforce the absoluteness of their statement, “…before the acceptance of an affirmative decision by the experimental subject there should be made known to him the nature, duration, and purpose of the experiment; the method and means by which it is to be conducted; all inconveniences and hazards reasonably to be expected; and the effects upon his health or person which may possibly come from his participation in the experiment. The duty and responsibility for ascertaining the quality of the consent rests upon each individual who initiates, directs, or engages in the experiment. It is a personal duty and responsibility which may not be delegated to another with impunity.”
In No Hurry
The FDA followed this up with an amazing display of inertia. In fact, it would be another decade before a judge (once again, it was the judicial system rather than congress or the agency) coined the term “informed consent” in a ruling against a doctor who paralyzed a patient with a procedure after not properly warning him of the risks.
It took two and half more decades, 1981 to be exact, for informed consent to be codified in U.S. federal law through coordinated policies of the FDA and the U.S. Department of Health and Human Services.
The most devilish of Devil’s advocates might argue that the war crimes occurred under a despot in Germany. So, why would a federal agency in the U.S. feel any pressure to react?
Well.. it is precisely what did happen on this soil that makes their lethargic approach to patient protection all the more jarring.
Lesson Learned
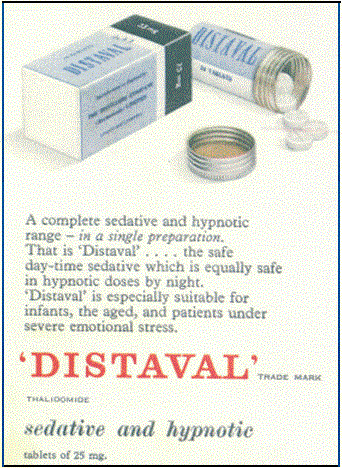
Around the same time a judge in California gave “informed consent” its name, the Merrell drug company pressured a young Pharmacology Ph.D. at the FDA to approve a new drug, but she refused, citing a lack of evidence. Despite their repeated attempts to strong-arm her, Dr. Frances O. Kelsey never signed off on thalidomide. Her tenacity likely saved tens of thousands of Americans from suffering horrific birth defects and earned her distinguished recognition from President John F. Kennedy. I wrote about these events in greater detail in my book, In the Name of The Pill.
The close call with Thalidomide left many in Congress more concerned than ever about ineptitude at the FDA. The resulting legislation, the Kefauver-Harris Drug Amendments, gave the FDA more control over the approval process and required the drug companies to report adverse reactions. The amendments also put a greater emphasis on proving the efficacy of a drug during clinical trials.
Not So Much
Undoubtedly, when Congress approved Kefauver-Harris, they recognized that the thing that made Dr. Kelsey’s story unique was not that she was being strong-armed by a drug company, but that she stood her ground.
In fact, the similarities (which would not become public until much later) are eerie when compared with the details of the approval of another potent drug, hormonal birth control. Dr. John Rock went on record to boast about how he browbeat the FDA into approving The Pill despite insufficient safety data.
Ironically, when the FDA later assembled a committee to investigate whether The Pill had ever been proven safe, it would be the greater emphasis on efficacy imposed by Kefauver-Harris that the committee chairman would contort to give a friendly nod to The Pill.
When Dr. Louis Hellman was appointed chairman of this FDA advisory board, his cozy relationship with the drug companies was not yet obvious. However, by the time the Nelson Pill Hearings rolled around in 1970, it was clear that Sen. Gaylord Nelson held a level of contempt for Dr. Hellman’s lack of ethics as a shill for the drug companies.
During the hearings, Sen. Nelson pointed out that, despite having issued a chairman’s statement that The Pill was “safe within the intent of the law (Kefauver-Harris),” Dr. Hellman had previously lamented that the committee had to come up with the “right” statement. They had to issue an opinion, but if they stated that the drugs were not safe, the FDA Commissioner might have to take them off the market. They needed some way to affirm that The Pill was safe enough, although their “scientific data did not really permit that kind of statement.”
Coordinated Entropy
There is a basic law of entropy, which states that any spontaneous process will tend toward greater entropy (disorder) over time. But, that is a spontaneous process. When it comes to medical research, the entropy seems to be engineered as a direct affront to law and order.
Anytime Congress or a regulatory agency tries to define a suitable order for their practices, the drug companies skate right up to the newly drawn line and begin testing it – first sticking a hand over the line and then a foot. Before you know it, they have Hokey Pokey’ed their way back into business as usual.
Consider that in the wake of Nuremberg, Dr. Gregory Pincus conducted hormonal birth control trials in Puerto Rico, in which the women were not told they were participating in a trial. Five of those healthy, young women died and were buried without autopsies because the doctors believed their little miracle pill could not have been a factor. And, when 65% of the women complained about the side effects, Dr. Pincus dismissed the complaints as psychological in nature because of the “emotional super-activity of Puerto Rican women.”
The history of shameful trials do not end with Dr. Pincus. In 1966, Dr. Henry Beecher cited dozens of examples of dubious medical practices to highlight the need for stronger protections. Shockingly, many of his colleagues ostracized him, calling his accusations a “gross and irresponsible exaggeration.”
Mounting Evidence
Examples from Beecher’s Bombshell, as it became known, included instances of doctors transplanting melanoma into healthy patients, withholding penicillin from soldiers, and infecting disabled children with hepatitis to study the disease.
Other well-known instances of less-than-ethical medical practice and research include:
Mississippi Appendectomies, where minority women were surgically sterilized without their consent.
Tuskegee syphilis study, a 40-year non-therapeutic “study” that documented the affects of untreated syphilis on black men in the deep south.
Milgram Experiment, a troubling study of obedience to authority where subjects believed they were administering shocks to people they had just met, potentially to the point of death, in order to satisfy the commands of an authority figure.
Whether these examples are the exception, the rule, or somewhere in between, they do confirm one thing. Medical professionals with no moral compass pose a threat from which we, the public at-large, need to be protected. The only solution is informed consent, without mitigation.
Same Difference
History shows us we need to be concerned about doctors who would push back against informed consent – even if their language says it is only relevant to matters of “minimal risk.”
Doctors developing The Pill were convinced they were only affecting ovulation, and would have, no doubt, argued it posed minimal risk.
Somewhere in the USA, a doctor injected hepatitis into children because, oh well, they’re already disabled.
Somewhere in the USA, doctors performed full hysterectomies on black women because they were deemed unfit.
Somewhere in the USA, a doctor intentionally transplanted cancer into healthy patients.
Dr. Beecher ultimately ended up with hundreds of examples of research gone wrong. He struggled to understand how these “men of science” could willingly “cause harm to patients with no possibility of benefit.”
It would be foolish to assume that doctors like this no longer exist. They personify the reason we should not be chipping away at the policies that protect us. Where will these “little” changes end? And that question is precisely why this new ruling is indeed newsworthy. The legislation was already amended to allow for an exception to informed consent in life-threatening situations, when “it is not feasible to get the consent of the subject or the subject’s representative.”
The new ruling also suggests that the FDA will be taking a very hands-off approach to defining what constitutes “minimal risk.” The power to waive informed consent requirements based on minimal risk now resides in the hands of Institutional Review Boards (IRBs), meaning that a board designated by the very institution wishing to perform the study will be interpreting the meaning of “minimal risk.” Conflict of interest much?
Maybe it is no big deal that they already eliminated protections for the most extreme cases, and now they are eliminating protections for the least extreme cases. Maybe it is no big deal that we are going to have to trust researchers to define what falls into that still-protected middle ground. But, maybe it is a big deal.
On another note, the quote at the beginning of this article comes from a song titled, Won’t Get Fooled Again.

The FDA approved The Pill despite it not being proven safe. Today, it has been linked to everything from blood clots and cancer to lupus and Crohn’s disease — and still has not been proven safe.
This book explores the medical and historical disconnects that brought us to this point.
Last updated on October 21, 2023 at 9:38 pm – Image source: Amazon Affiliate Program. All statements without guarantee.
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
Image by jannoon028 on Freepik








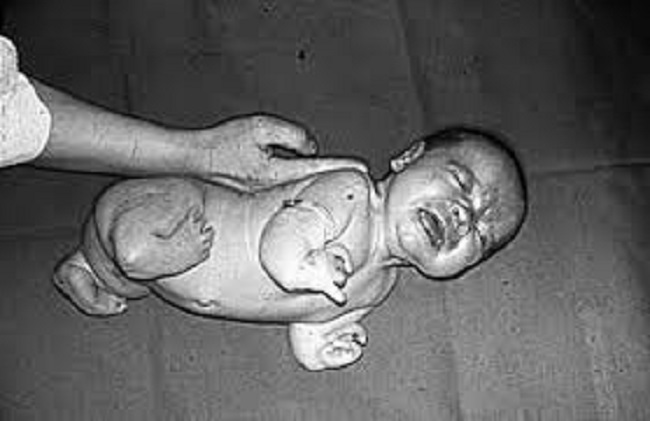





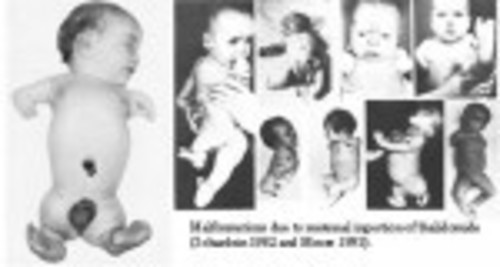
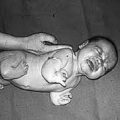
 DES is not something of the past. People who have been exposed to this drug years ago are battling with health issues and fighting for their lives as I’m writing this blog post. Who knows what health problems the grandchildren of the mothers who were prescribed this drug will have to deal with as they grow up. I want my daughters to receive adequate medical care and monitoring if they ever have to suffer the consequences of this drug. This is why together with my husband we support the great work done by the very few International DES Action Groups who are providing valuable information and are advocating for the DES victims.
DES is not something of the past. People who have been exposed to this drug years ago are battling with health issues and fighting for their lives as I’m writing this blog post. Who knows what health problems the grandchildren of the mothers who were prescribed this drug will have to deal with as they grow up. I want my daughters to receive adequate medical care and monitoring if they ever have to suffer the consequences of this drug. This is why together with my husband we support the great work done by the very few International DES Action Groups who are providing valuable information and are advocating for the DES victims.