Uterine morcellation, the surgical technique that involves using a morcellator device to mechanically chew up larger pieces of uterine tissue, tumors and fibroids into much smaller, more easily removable pieces of tissue, has been a mainstay of laparoscopic gynecological surgeries, especially hysterectomy, since the mid nineties. It was only recently, when a high profile physician was herself injured by morcellation, did the dangers of morcellation come to the attention of the media and the broader medical community. One has to wonder, who the heck thought grinding up potentially diseased tissue and spreading those diseased cells, however inadvertently, within peritoneal cavity was a good idea in the first place? Really, how in the world did these devices get approved and so readily adopted into medical practice?
FDA Clearance of Morcellators: No Safety Data
The first electromechanical tissue morcellators were cleared for sale in 1995 FDA’s 510k process. The 510k process is used for devices that are considered substantially equivalent to medical devices or tests already on the market. The 510k clearance is not approval per se, but a quick step around the approval process. It allows the manufacturer to go straight to market with the product, after a 90 day waiting period. No clinical trials are required, no safety data are required. According to the Project on Government Oversight blog:
“…the 510(k) process doesn’t evaluate anything about a device except whether it is substantially equivalent to previous devices. Thus it can’t really ensure the safety and effectiveness of these devices.”
Morcellators were cleared for sale via this process of suggesting that they were no more dangerous than the laparoscope itself. Since 1995 the FDA has cleared about two dozen electro-mechanical morcellators, all via the 510k process. In essence, these devices came to market without safety data. Indeed, there were no published studies evaluating the safety or efficacy of these devices for almost the first 10 years of their use, although case studies began emerging in 2002. How was that possible?
Adoption of Morcellation in Gynecological Surgery: The Business Case
Over half a million women in the US have hysterectomies annually. In fact, every 10 minutes, 12 American women lose their reproductive organs, every day of every year. Worldwide, the numbers are equally staggering. By the age of 60, one third of all women will have had a hysterectomy. Between 70-90% of hysterectomies are deemed medically unnecessary. Worse yet, an estimated 73% of hysterectomies include the removal of the ovaries, a procedure akin to male castration with all of the concomitant side-effects one might expect when critical, hormone-producing, organs are removed. Unnecessary hysterectomies are big business for hospitals and surgeons. It is within this landscape that morcellators found their market.
Morcellation is a no-brainer within the ever-expanding business landscape of hysterectomy. Considering up to 90% of hysterectomies are medically unnecessary in the first place, it is not difficult to see how decisions about surgical techniques and instruments might be more skewed toward efficiency than safety. And of course, from a purely mechanical perspective, grinding large matter into smaller pieces makes quick work of tissue removal from the ever decreasing size of excisions. Morcellation with laparoscopic or robotic hysterectomy is more efficient. No doubt, that is how these devices were marketed.
From a biological perspective however, morcellation makes absolutely no sense whatsoever. How did so many physicians, so easily disregard core concepts of human biology – that spreading cells means spreading disease – in favor of the latest gadgetry? The potential risks of mechanical tissue morcellation could not be clearer. According to one of the first studies (2012) to address uterine morecellation:
“In order to remove these bulky lesions from the abdominal cavity through laparoscopic ports the tumors must be morcellated. This technique involves fragmenting the lesion such that it can pass through a small incision (i.e. the laparoscope port itself). Originally performed by hand with the assistance of a laparoscopic scalpel, newer methods involve the use of power morcellators, devices designed to draw the lesions into a whirling blade, which then generates small (approximately 1 cm diameter) cores of the lesion, capable of being removed through the port incision. The velocity with which these blades spin has been associated with dispersal of microscopic tumor fragments, thus potentially seeding the peritoneum with small pieces of both neoplastic and non-neoplastic material. This phenomenon is compounded with the fact that some morcellated tumors are not benign.”
Indeed, the research shows the risk of metastatic cancer post morcellation is up to 9 times higher than when non-mechanical surgical techniques are used. Case reports show the possibility of spreading endometrial implants and parasitic myomas post morecellation.
Rate of Morcellation and Risks
Data are scant on the percentage of hysterectomies using morcellation and even more sparse on the adverse events associated with these devices. Some estimates suggest 11% of ~600,000 hysterectomies annually use morecellators – about 66,000 women per year. The risk of morcellation of an occult tumor is believed to between one in 400 and one in 1000 women. The risk for dispersal non-cancerous, but diseased tissue such as endometriosis or parasitic myomas is unknown. However, with such spotty reporting on these devices and this technique, it is difficult to calculate the real risk or even the real use patterns. The number of women potentially harmed by morcellation could be much larger.
The Morcellation Debacle
In essence, mechanical morcellation seeds cells in the abdominal cavity. If those cells are diseased or cancerous, the results can be deadly. And yet, this device was cleared by the FDA and adopted by surgeons with nary a question of its obvious risks – risks that could be presupposed based upon basic principles of biology. Of course, mechanical, high speed tissue morcellation would spread microscope cells. Of course it would. How could this not be recognized up front? How did it take until 2014, 19 years on the market, before the medical societies and the FDA recognized the dangers? More so, even though there were case reports beginning in 2002, evidence that the manufacturer was warned of its dangers in 2006, a large study in 2012, it wasn’t until a prominent physician was injured herself, and her husband, also a prominent physician, exposed the dangers publicly that the respective medical societies and the FDA recognized these dangers and felt compelled to issue statements of risk. To say this is an egregious lack of oversight does not begin to capture the across-the-board levels of ignorance and incompetence associated with the adoption of this procedure.
Sign a Petition to Stop Morcellation
Participate in Research
Hormones MatterTM is conducting research about hysterectomy. If you have had hysterectomy, please take a few minutes to complete this important survey. Then share the survey link with all of your friends. These data could save the life of another women. The Hysterectomy Survey.
To learn more about our research, click Take a Health Survey and sign up for our newsletter for updates on the latest research and new surveys.
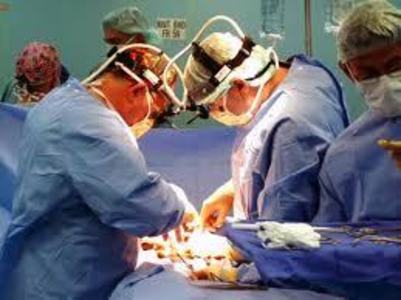










I have been suffering from Power Morcellators side effects as i have had fibroid surgery. Now i am looking forward to consult an attorney so that they can take action against the hospital and the Power Morcellators manufacturer. I have consulted with a morcellator-attorney. Can you help me regarding my problem?