A Rant
Women who have been given Lupron (leuprolide) and the other GnRH agonists and antagonists and women who have had their ovaries removed are thrust violently into menopause. Overnight. There is no gradual decline of ovarian hormones that allows for molecular adaptations to the new state of chemical senescence. No, none of that. Just the chemical trauma of the loss of hormones.
The experience is akin to castration; something that should never be considered as a viable treatment option for any benign disease process, especially in young people. Yet millions of young women and children (Lupron for precocious puberty, gender dysphoria, and now autism) are prescribed these drugs and undergo these procedures annually without as much as the slightest recognition that there might be negative sequelae. Indeed, women are told routinely that what they experience with Lupron, its analogs, or upon oophorectomy is not real, that it does not exist, and that their symptoms are no more than some form of psychosomatic stress – the long vestige of Freudian acquiescence called hysteria. It is not. The symptoms these women experience is real and directly related to how the loss of estradiol and other ovarian hormones damage the mitochondria.
Some Mechanisms
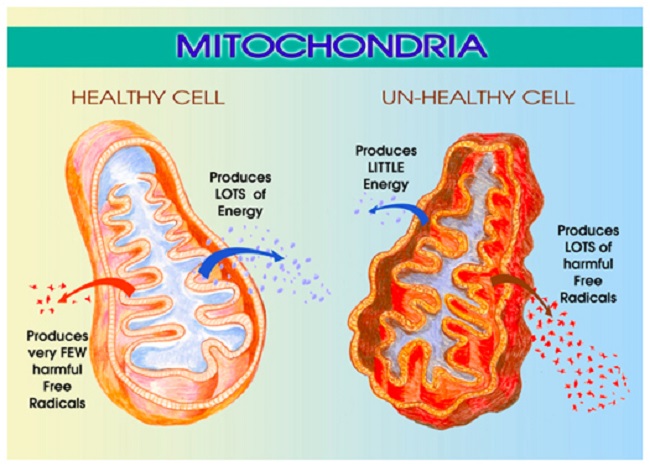
Estradiol regulates mitochondrial energetics via multiple mechanisms both directly and indirectly. I have written about the mitochondrial damage provoked by the loss of estradiol previously, see here, here and here. In brief, the loss of estradiol fundamentally changes the shape and functionality of the mitochondria, effectively disabling not only their ability to produce ATP (cellular energy), but also, reducing their capacity to perform the myriad of vital functions for which the mitochondria are responsible. The reduction of ATP is alone sufficiently damaging and I would argue the root of all disease. Without sufficient ATP all sorts of vital functions grind to a halt, but when one considers that the mitochondria are responsible for managing immune and inflammatory signals, the production of steroids, the sequestration of Ca+ (cell excitability), the removal of toxicants, both endogenous and exogenous, and even cell life/death cycles, the logic behind using a form medically induced castration as a treatment for any disease becomes suspect.
For all the young women who suffer through the loss of estradiol, either via Lupron, its analogs or via oophorectomy, the damage is believed to be irreparable, mostly because there is neither recognition of the ill-effects nor any research into possible recovery options. Some of the damage may be permanent, unfortunately. It is impossible to tell at this point. However, and this is a big however, the human organism is remarkable in its ability to heal and sustain life despite our best efforts to the contrary. I believe strongly that the body can recover from just about anything, save except death. One just has to give it what it needs to heal. That is, if we provide the core substrates, the appropriate fuel sources and nutrients and if we remove the toxins, healing can occur. It will not be quick and it may not be complete, but it will happen. This is where basic research comes into play. Though not ideal, it can guide us. I will explain, but first, let us review some components of mitochondrial illness.
Understanding Mitochondrial Damage
Mitochondria are central to cell survival, and thus, our survival. Mitochondria take the foods we eat and through a series of enzymatic reactions convert the food into chemical energy called ATP. ATP fuels everything. Without ATP, cells struggle to function, become hypoxic, protein synthesis and repair processes falter producing aberrantly folded proteins, until the damage becomes too great, overwhelming their capacity to function. Cells die, tissues and organs die, and eventually, we die. Before we die, however, a whole host of seemingly random and complex illnesses and symptoms emerge as a direct result of the diminished ATP. That is, in an effort to keep us alive, the mitochondria and the cells in which they reside initiate survival cascades, key among them, inflammation and immune reactivity. These cascades, if left unchecked, evoke even more damage, illness, and eventually death. Yes, mitochondrial damage can precipitate death or a life so painful that death may seem preferable. The pain and suffering these women experience is real.
What Causes Mitochondrial Illness?
Mitochondrial illness can be initiated via a whole host of interacting variables. Genetics play a role, but so too do epigenetics – environmental stressors that activate or deactivate genes. Diet is a huge contributor. Too much sugar, processed food, alcohol and not enough nutrients are key variables determining mitochondrial illness or health. Nearly every, if not every, pharmaceutical damages mitochondria via one mechanism or another. All environmental and industrial chemicals damage the mitochondria. Hormones too influence mitochondria. Estradiol is top among them, but likely not the only hormone influencing mitochondria, only the most frequently studied.
Presentation of Mitochondrial Illness
Mitochondrial illness breaks all the rules of modern medicine. The symptoms are highly varied and individual. They do not fit into our discretely compartmentalized view of illness, and how could they? Mitochondria are the engines of every cell in the body, powering all life sustaining functions, from the brain and nervous system to the heart, the GI system to the musculature and everything in between. So when the mitochondria struggle, we have symptoms everywhere, in every compartment of the body, but exactly how those symptoms present is as individual as we are.
When the mitochondria are struggling, the systems that require the most energy are taxed most, but depending upon the individual’s genetic/epigenetic and nutritional liabilities, all sorts of weird symptoms may emerge due to the lack of ATP, the survival cascades initiated, and the other mitochondrial functions that now struggle. In that regard, even folks with the same constellation of genetic variables, express their symptoms differently. The wild variability in symptom expression makes mitochondrial disorders simultaneously the most difficult and the easiest to diagnose; most difficult if one subscribes to a compartmentalized, organ specific form of medicine and easiest if one looks to root causes. At the root of every disease process, whether cause or consequence, are struggling mitochondria. All disease begins and ends in the mitochondria. As such, unless and until the needs of the mitochondria are addressed, healing cannot occur. The flipside, of course, is if we support mitochondria, healing becomes possible.
Mitochondria, Estradiol and the Problem with Medically Induced Menopause
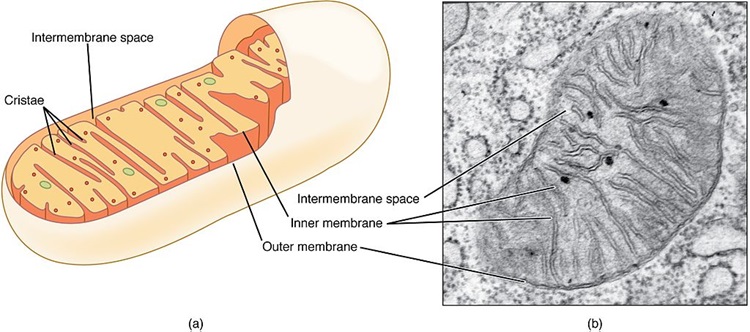
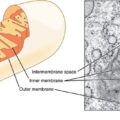
As mentioned previously, when the mitochondria are deprived of estradiol, the membranes surrounding the mitochondria become deformed. The increased permeability of these membrane causes all sorts problems, but top among them, the transfer of nutrients is less efficient. Mitochondria require at least 24 vitamins and minerals to efficiently convert the food we eat into ATP. Though it is not clear what happens first, whether the enzyme machinery responsible for processing ATP diminishes heralding the increased permeability and deformation of the mitochondrial membranes or whether the loss of estradiol deforms the membranes first and that then results in a loss of ATP, it is clear that the loss of estradiol severely constricts mitochondrial functioning. In both cases, the loss of functionality initiates the survival cascades, and what many call the death spiral, begins.
Clues to Recovery
The easy answer would be to replace the lost estradiol. The problem with that, however, is that the ovaries synthesize a whole host of hormones, not just estradiol, and our ability to replicate those hormones in the appropriate concentrations is just not there yet. Nevertheless, working with a physician or pharmacist experienced in bioidentical hormone replacement may be helpful, at least in the transition period while the body is adapting to the new state of chemistry. The use of synthetics, however, are likely to do more damage than they are worth.
I believe a more prudent approach would be to tackle the health and efficiency of the mitochondria themselves; an approach that had it been undertaken prior to the use of Lupron or oophorectomy may have been able to reduce the impetus for these procedures in the first place. In fact, these recommendations are not specific to Lupron or oophorectomy, but carryover to any type of mitochondrial illness.
Vitamins, Minerals, and Diet
Considering the mitochondrion’s primary purpose is to convert the food we eat into life sustaining ATP, the simplest and most often ignored component of recovery, is to feed the mitochondria what they need to function and avoid the stuff they do not. In other words, eat well and avoid chemical toxicants. In practice, however, this seems to be exceedingly difficult for most of us. Decades of processed food marketing has skewed our preferences away from ‘real food’, and in many ways, disconnected us from the purpose of eating – to fuel our bodies.
Mitochondrial Nutrients to Rescue Mitochondria
There are 24 of vitamins and minerals required to power the mitochondrial machinery responsible for converting food into ATP. Ensuring an adequate supply of nutrients is critical to repairing mitochondrial damage, perhaps more important than any other aspect of recovery. Top among them are thiamine and magnesium. We have written about this extensively on Hormones Matter and in our book. Thiamine is the gatekeeper to the mitochondrial factory, involved in the initial enzymatic reactions required to convert consumed carbohydrates, fats and proteins into ATP and just about every enzyme reaction throughout the process. More so than any other nutrient, thiamine is critically important to mitochondrial functioning, but its deficiency is least likely to be recognized, even though it causes a host of serious disease processes including death. Magnesium is an essential cofactor for thiamine. Deficiencies in thiamine and magnesium may well account for the vast majority of modern illnesses.
The other B vitamins are also important, as are a variety of minerals. Which ones and in what doses are required for recovery is individual, but often, the dosages are far higher than what is proposed by the RDA and in combinations that are not typically found in standard, over-the-counter (OTC) supplements or even in marketed mitochondrial cocktails, though some of these products are considerably better than the OTCs. In the case of mitochondrial recovery, vitamins and minerals are used at pharmacological doses in order to kick start, and in many cases, compensate for the increased nutrient demands caused by the damage and/or by genetic or epigenetic variables. For individuals with severe deficiencies and/or poor absorption, repeated intravenous vitamins/minerals may be warranted. The details of dosing are covered in our book.
The Mitochondria Diet: Eat Real Food
The second component of healing mitochondria involves diet more broadly. That’s right, what we eat can harm or heal us. Processed foods must be eliminated, as should alcohol and other toxicants. Diets should be high in protein and good fats and low in carbohydrates, and to the extent feasible, organic. Why proteins and fats? Chemistry. Health demands that protein synthesis outpace protein breakdown. That requires a ready supply of protein from the diet and the appropriate nutrients listed above to process them. There is much debate about how much protein, but broadly, and depending upon level and type of illness, one must consider.08 – 1.5kg of protein per kg of weight per day. In research of critically ill patients, higher protein consumption (~1.5kg/kg/day) is associated with better outcomes. With our current predilection for excess carbohydrates, most folks are nowhere near even the lower end of these requirements and that creates a barrier to healing.
The integrity of mitochondrial and all cellular membranes require fatty acids. For women recovering from the loss of estradiol, and the subsequent deformation of mitochondrial membranes, a diet poor in essential fatty acids would be doubly debilitating. Indeed, for most people, an increase in fatty acids would improve health considerably. Fatty acids are also a key fuel substrate for mitochondrial ATP (which just so happens to be thiamine dependent as well).
In general, lower carbohydrate intake is warranted. High carbohydrate intake exacerbates thiamine deficiency and in fact, by itself, with no other risk variables, can induce thiamine deficiency. Researchers from USC have shown that immediately following the loss of estradiol, brain glucose transporters begin to downregulate forcing the brain into ketosis. That is, at least in mice, the loss of estradiol forces a metabolic shift towards using ketones as the primary fuel source to produce ATP. This means that our traditionally carbohydrate dense diets may not meet the brain’s energetic demands when estradiol is absent. Granted, this research used mice and is preliminary, but it suggests an additional route to healing post Lupron and oophorectomy would be to increase dietary fat. Ketosis may be an option to repair mitochondrial damage.
Having said that, there are disorders of fatty acid metabolism which make lower carbohydrate diets very difficult and sometimes even dangerous. Full blown manifestations of these disorders usually occur in infancy or childhood, but more subtle manifestations brought on by single nucleotide polymorphisms (SNPs) in key genes may remain somewhat latent until triggered. Anecdotally, the inability to metabolize fats seems relatively common among the folks who interact with us, manifesting in what can only be described as energetic collapse post ingestion of fats and proteins. In the cases where SNP analyses are available, the difficulties are related to what have been traditionally considered ‘rare’ variances in key enzymes. Nevertheless, the parameters of these recommendations hold true for most people. Eat real, nutrient dense foods. The specifics of the diet, like the specifics of the nutrient replacements is all that varies.
Avoid Toxicants
Finally, with poorly functioning mitochondria, taxing these organelles further by ingesting the chemically laden foodstuffs produced by conventional agriculture and livestock practices makes healing that much more difficult. To the extent dietary toxicants can be avoided, they should. Similarly, since most, if not all medications damage mitochondria and deplete vital nutrients by one mechanism or another, medication use should be evaluated thoughtfully assessing potential benefits against likely risks.
Is it Really That Simple?
Yes and no. In many ways, it is very simple. Feed the mitochondria and they will do the rest, but in other ways, it is not so simple. Diet is absolutely critical, but mitochondrial recovery also includes using supplements at pharmacological doses, sometimes intravenously, to rescue damaged mitochondria and induce a more favorable mitochondrial replication process. This is difficult for some folks to access. Few physicians have any background in this and many simply do not believe that mitochondrial damage exists or evokes illness. Worse yet and despite evidence to the contrary, many physicians are steadfast in their belief that pharmaceuticals have no bearing on mitochondrial function, and thus cannot possibly be responsible for these illnesses. This means that to the extent pharmaceuticals do not treat and often make matters worse in these cases, fuel to the ‘it-must-be-hysteria-or-psychosomatic’ fire is added. In many ways, the biggest stumbling block is the notion that mitochondrially-mediated disease processes exist at all. A close second, is that nutrients can be used to recover mitochondria and in many cases, override even genetic defects.
Making matters even more complicated, there is no one-size-fits-all diet or supplement protocol. While it is true that thiamine is critical and must be addressed if there is any hope of recovery, everything else downstream must be considered on an individual basis. We each carry a host of unique genetic, epigenetic and environmental exposures that combine to make a complicated chemistry; one that no one wants to untangle and few have the skills to do so. As a result, it is much easier to dismiss the symptoms as psychosomatic and prescribe an antidepressant or other pharmaceutical. I would also mention, even among those of us who have a background in this stuff, we do not know everything and cannot know everything. Untangling these patterns takes time, effort from the patient, lots of research from both parties, and a fair degree of trial and error. Finally, recovery is neither a straight line nor an absolute. There are setbacks, sometimes serious ones, and health has to be actively managed from this point forward. Even with all of these complications, however, better health is possible if one tends to the mitochondria.
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
This article was published originally on August 6, 2018.