If you were seriously ill in ancient Babylon the priest-physician would have asked you to breathe into a sheep’s nose, whereupon the sheep was slaughtered to get a ‘reading’ of your illness and prognosis from your liver. It was believed the gods revealed their intentions through the master organ of the liver. – paraphrased William Snively, The Sea Within
Oxalosis Is About Poor Liver Elimination and Not Solely Diet Related
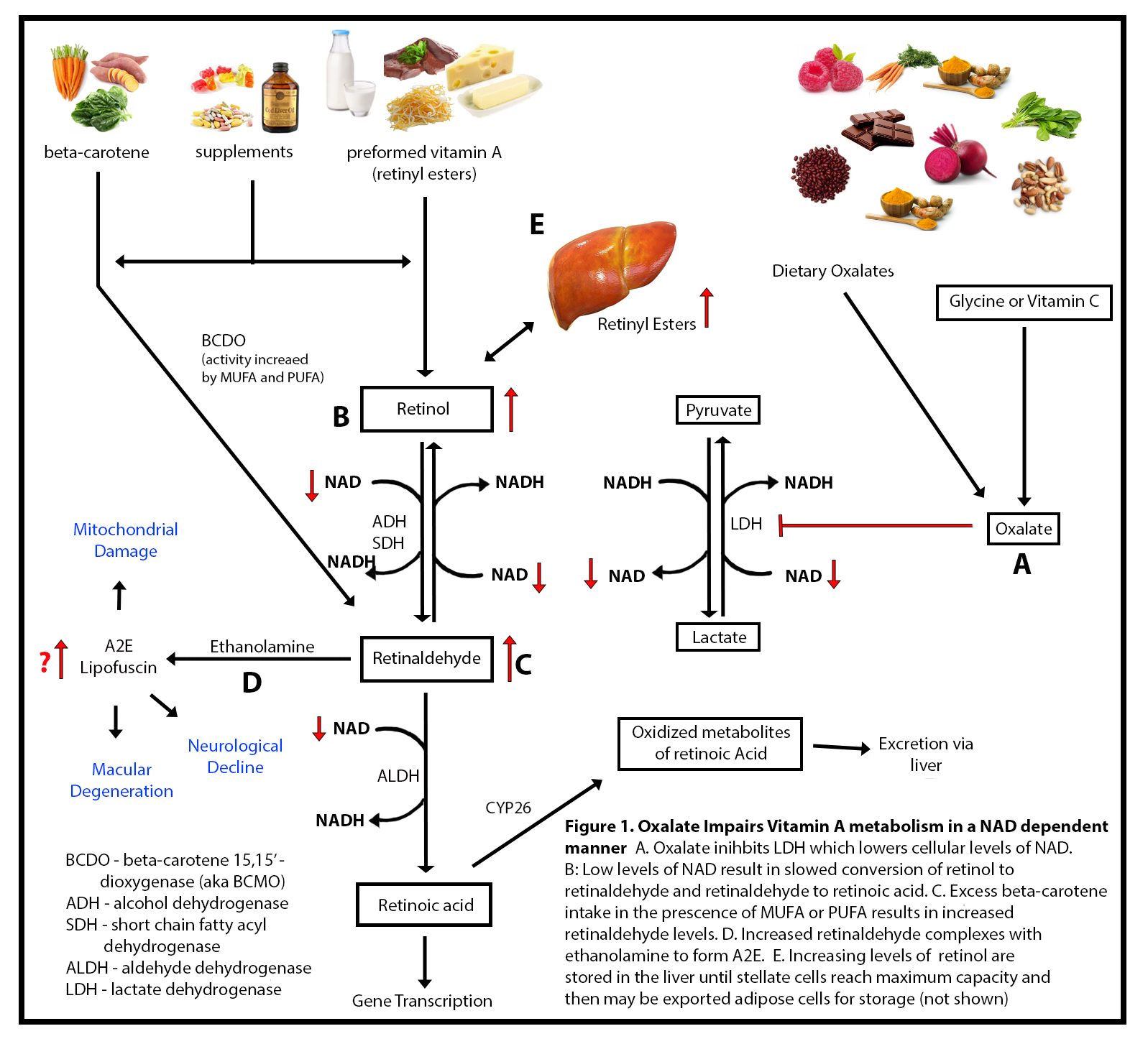
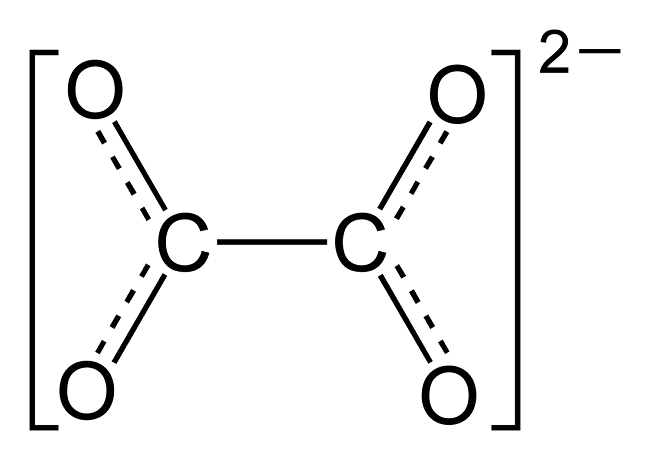
There is a truism in the sociology of occupational knowledge: “If all a doctor has is a hammer, then everything becomes a nail”. All modern medicine relies on overkill dosages of drugs or surgery as the standard of care (Jennifer Daniels, MD, The Lethal Dose, 2013). There are no drugs or surgery that can alleviate the symptoms of poisoning from a natural pesticide found in plant foods but also produced in the liver. With this professional void, treating oxalosis has mainly been left to nutritionists. So, if you ask a nutritionist about oxalates, you will get a predictable answer to reduce oxalates in your diet. This is insufficient because most oxalates are produced by poor metabolism in the liver. Oxalate is a natural pesticide found in plant foods, but also is internally produced mainly from fungus and vitamin C (see How Oxalates Ruin Your Health). Oxalosis manifests as a syndrome of three main symptoms: oxalate crystals in tissues and kidney; histamine, mucous attack in nasal passages; and pseudo-gout mainly from acidity and eating cooked meat.
With Oxalosis, You’re Lost in an Ocean Without a Compass
I make no pretense to have expert knowledge or training on this issue beyond my personal knowledge of finding some solutions to my own oxalosis. Indeed, my own vitamin guru brother kept pushing on me mega doses of Vitamins C and D, avoiding calcium and iron, as a panacea for everything. Much later, I found that “health foods”, such as vitamins C and D, spinach and a plant diet, and the avoidance of calcium and iron were contributing to manufacturing oxalates in my liver.
Only after hitting a wall of brain fog, fatigue, pseudo-gout, nasal mucous attacks, extreme lack of thyroid hormone, pins and needles on the bottom of my feet, and crystals popping from my eyes, did I seek medical consultation for these symptoms. After several futile consultations, I found that doctors don’t know anything about oxalates. Finally, a naturopath doctor gave me an office blood blot test and stated I had oxalates. The only knowledgeable people I could find about what to do with oxalates was from a self-help group at TryingLowOxalates.io and the Hormones Matter.com website. I won’t bore you further with my medical system merry-go-round story that all those with oxalosis go through and instead will try and relate what I have learned to date, albeit all errors are my own.
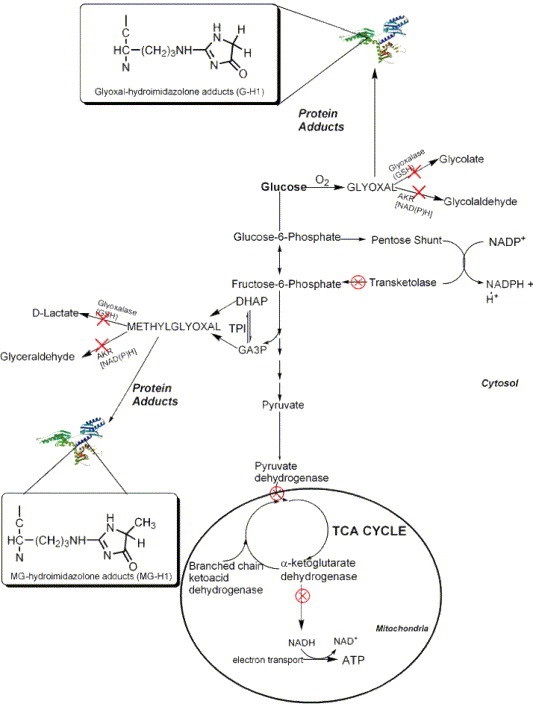
Endogenous Oxalates Mainly Begin in the Liver
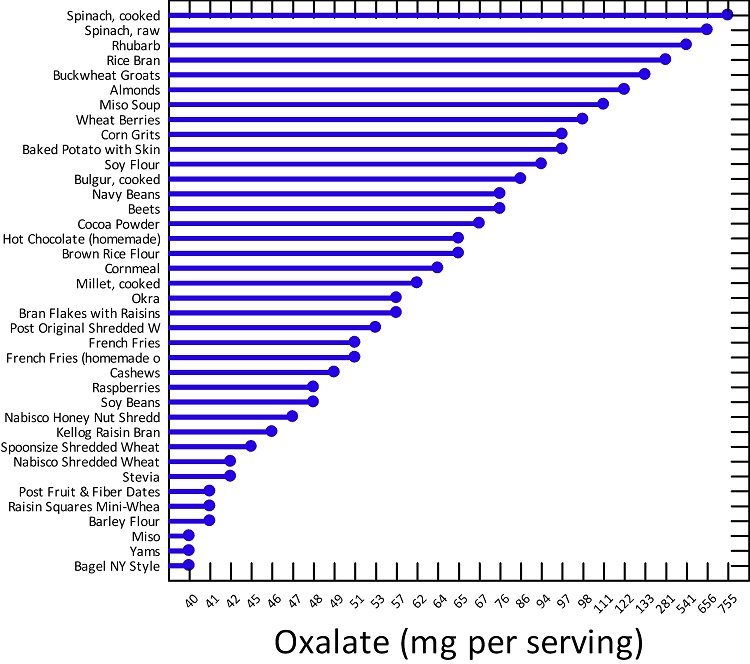
First, I learned that calcium is needed as a co-valent chemical binder to oxalate before meals to eliminate oxalate through the bowel or oxalic acid through with urine through the kidney. Secondly, I learned that plant foods like spinach, almonds, chocolate, and soy and were loaded with toxic levels of oxalates, a natural pesticide that protects plants from insects, worms, and herbivores. I also learned that high doses of synthetic Vitamin D (10,000 mg/day as a steroid) without Vitamin K2, could also lead to kidney stones and oxalosis. An iron deficiency may come into the picture as discussed below.
My learning curve with oxalosis ramped up when I read the 1950’s book Food is Your Best Medicine by Henry Beiler, MD. How could food be the best medicine when it was “healthy plant food” that was my pathway to chronic disease and eventually contributed to a minor heart attack (elevated troponin protein only)?
Beiler says all disease is a failure of the elimination systems (defecation, urination, expiration, perspiration, hydration, inflammation), not the immune system, in our body. The “master cylinder” elimination organ is the liver, which filters solid wastes. The kidney excretes wastes suspended in fluid such as oxalic acid. The lungs rid the body of C02 by coughing. Hydration dilutes oxalic acid in the gut making it less likely to form crystals. Perspiration expels toxins through sweating or skin rash, and inflammation is the depositing of toxins inside the body to quarantine them (e.g., cancer). Paraphrasing Beiler:
When the liver is congested it can no longer perform its eliminative function and waste matter is thrown into the bloodstream. Toxic blood must discharge its toxins, or the person dies (sepsis). So, nature uses ‘vicarious elimination’ of having the lung and kidney help-out in eliminating toxins and poisons in the liver. Fluid accumulates in the lung because the liver can’t filter food and toxins at the same time.
This is evidently how respiratory infection can begin without any airborne contagion from a virus.
He goes on to argue that toxic blood gets to the lung through the arteries and then permeates into the lung by cellular membrane diffusion in balloon-like alveoli sacks. For oxygen to get distributed throughout our body it first needs a transporter of red blood cells to carry oxygen-binding molecules called hemoglobin. Dietary iron is the building block of hemoglobin and is carried throughout the vascular system to bone marrow which is the farm for making red blood cells. Oxalates can bind to iron and subsequently lead to chronic anemia. So, oxygen production begins in the gut not the lung. Iron anemia is a predictor of poor outcome from lung infection. The oxygenated red blood cells then are carried throughout the body by the flow of blood.
He contends that cancer is the quarantining of toxins in tumors that switches cellular respiration from oxygen to sugar and oxalates can cause cancer in breast tissues. And finally, he says that heart disease occurs when there is a failure to get enough oxygen-rich blood to heart muscles (heart attack from irregular heartbeat arrhythmias) or to the brain (stroke).
Killer Proteins and Oxalosis
I shall try to show how oxalosis is related to excess unmetabolized protein in the human body. Beiler asserts “proteins can be body killers if we are not watchful of our diet”. Protein is necessary to grow and repair the body. However, poor metabolism with age often leads to making metabolites (non-nutrients) from fat, protein, or carbohydrate (not just from dietary oxalate consumption). Excess protein is one of the main sources of acidosis, which can be life threatening in the blood stream but not in the urine or saliva. Protein can change into fat or carbohydrate, but fat and carbs cannot morph into protein (Beiler).
One of the major symptoms of oxalosis is histamine or mucous in the nasal passages, for which there are no known explanations as to its mechanism of action with oxalates. “The more proteins are heated or cooked the more the colloidal form is changed”, the more mucous develops in the nasal passages. Unmetabolized proteins are expelled through a process called “vicarious elimination” through the nasal passages as mucous and or by diffusion into the spinal cord. Beiler adds:
When the adrenal glands are strong, they try to compensate for the liver’s failure by super-oxidation (requiring iron); this gives rise to increased kidney function. When both the kidneys and the liver become exhausted, the toxemia climbs to a higher level and often necessitates an attempt at vicarious elimination through organs that not normally excrete proteins.
For Beiler, oxalates are implicated in many cancers and heart disease where oxalate crystals in heart tissues or arteries potentially lead to fatal heart arrhythmias.
How This All Connects
My thesis is that internally produced oxalate is systemic and organic. It mainly manifests oxalosis (oxalate poisoning) and is produced in the liver, not solely from dietary oxalate, which can be managed with modest palliative measures. Oxalosis comes from poor elimination in the liver and not entirely from the food we consume. It is an interaction between the two in a feedback loop. I believe that the conventional approach to oxalosis, e.g. reducing the consumption of high oxalate foods, does not significantly reduce internal oxalate production, and thus, is merely palliative.
The motivation for this paper is that I could find no satisfactory explanation of the mechanism of action for endogenous oxalate other than vague references that it is produced in the liver, while dietary oxalosis happens in the kidney. Hereinabove I have hypothesized a plausible explanation of oxalosis from the liver from poor protein metabolism and oxidation. Since science is not just about finding evidence, but also about attempting to falsify a hypothesis, I am throwing this hypothesis out there for open refutation and clarification.
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, and like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
Image by Ray Shrewsberry • from Pixabay.