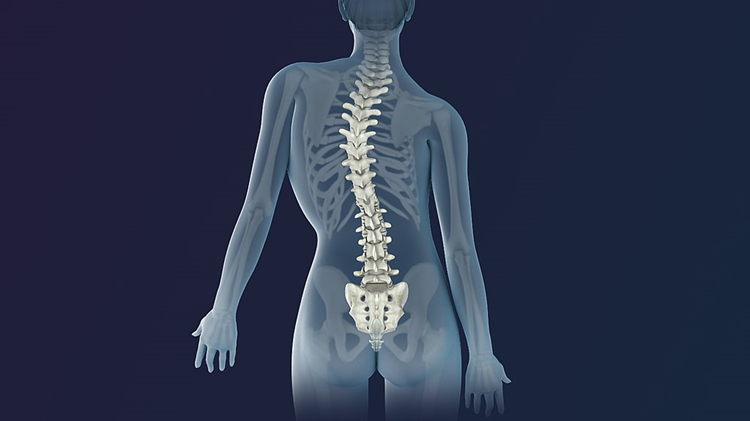
As everyone knows, the spinal column is made up of a series of bones known as vertebrae. Like bricks in a column, they collectively support the entire body in an upright position. Their anatomy is very complex because, collectively, they have to allow for the spinal column to bend in every direction without parting company with each other. Sometimes a child develops a permanent bend in the spinal column known as scoliosis. It usually develops relatively slowly and is usually watched by anxious parents as it becomes more severe. Anyone reading this who has had experience of it will know that the treatment is very advanced surgery in which the spinal column is straightened and supported with steel rods. This post is to try to explain why scoliosis happens and how it may possibly be prevented.
Brain Symmetry
Most people know that the left half of the body is controlled by the right side of the brain and vice versa. The reason for this asymmetry is unknown. However, asymmetry appears to be pretty important with many aspects of brain function. For example, I collected 17 patients, in each of whom their respective blood pressure was totally different in the two arms. The difference was so great that I imagined such an individual, when visiting a physician, would leave the office with a blood pressure pill if the pressure had been measured in the arm on the higher side, and without it if it had been taken in the arm on the low side. As most people know, the blood pressure is taken almost invariably in only one arm. The blood pressures in my 17 patients were compared with healthy controls. Although the pressures of controls varied only slightly in the two arms, the difference was extremely obvious when compared with the patients.
The asymmetry in the patients was greatly exaggerated, whereas in the controls it was minimal. This asymmetry is capable of increasing in direct relationship to loss of efficient metabolism in the control mechanisms in the brain. The loss of efficiency may be due to genetic effect or long-term malnutrition. These 17 patients had many symptoms, indicating that their autonomic nervous system was compromised because of poor oxidative metabolism. The symptoms responded to treatment with nutritional elements.
Dysautonomia
We have two nervous systems, known respectively as the voluntary and autonomic. The voluntary system enables us to use free will, whereas the autonomic is automatic and organizes brain/body functions that we cannot control voluntarily. The prefix dys means abnormal, so dysautonomia refers to abnormal function of that system. This can be due to genetic influence or from metabolic changes related to poor diet. One of the abnormalities that can occur is that the normal mild asymmetry in autonomic control can become exaggerated, giving more power to one side of the body than the other. Under normal healthy conditions, this asymmetry is much less marked. In fact, it is well known that all of us have some degree of asymmetry. One foot may be slightly bigger than the other or one eye may be a little bit more closed than the other. The autonomic nervous system is deployed to every part of the body and is the messenger system by which the brain controls all the organs that together, create body functions.
What Has This to Do with Scoliosis?
The spinal column is lined by very strong ligaments and muscles. It is these muscles that enable us to bend in every direction. However, those muscles are kept in what is called constant tone. Notice the hardness of the muscles in the low back. They feel to the touch like steel almost. This is because they are kept in tone as a permanent support. This tone is maintained through the autonomic nervous system by constant signals from the brainstem to the muscles surrounding the spinal column. The voluntary system can overcome the autonomic signals to enable us to bend as we wish. Asymmetric signaling to the muscles on either side of the spinal column occurs in health but may be only slight. If however, control mechanisms in the lower part of the brain are metabolically inefficient, asymmetric signaling evidently increases. If the tonic signals have exaggerated asymmetry, the tone of the muscles on one side of the spinal column will have stronger signals than on the other side. This explains why the scoliosis occurs gradually, but we have to realize that the real cause of the disease is because of the exaggerated asymmetry in the autonomic nervous system. In other words, scoliosis can be a disease of the autonomic nervous system as well as from genetic changes in the vertebral column.
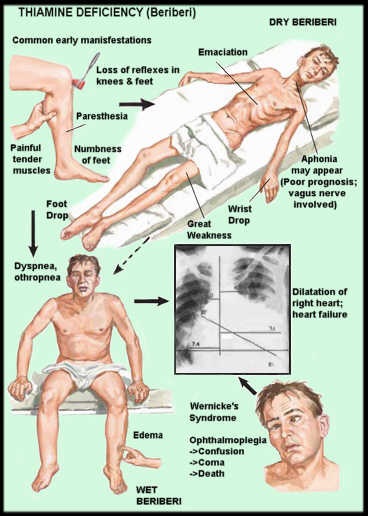
An experimental treatment for scoliosis was reported some years ago in which electrodes were attached to the weaker muscles on the convex side of the scoliosis and tonic electrical signals attempted to straighten the spinal column. I do not know what happened to that experiment, but it seems to me that scoliosis should be treated by preventive treatment of the dysautonomia. The patients with the asymmetric blood pressures all had evidence of dysfunctional oxidative metabolism in the lower part of the brain, an effect that can be produced by thiamine deficiency. All of them were treated by nutritional therapeutic measures with variable degrees of success. Quite a few of them, but not all, had thiamine deficiency as the underlying cause. It was concluded therefore that it was oxidative dysfunction, for any cause, in the brain that was the underlying cause of the exaggerated asymmetry, whether this was genetic or nutritional in origin. They responded to a number of intravenous infusions of water-soluble vitamins, all of which contained thiamine. The control mechanisms of the autonomic nervous system in the lower part of the brain are particularly prone to develop thiamine deficiency. Therefore this is an important cause of inefficient oxidative brain metabolism.
Prevention
As far as I know, prevention of scoliosis has never been attempted and what follows is therefore a hypothesis based on some evidence. It may well be that nutrition of the mother in pregnancy can induce faulty metabolism in the fetus that may deploy its effect in many different ways. We now know that thiamine deficiency is common in pregnancy and most of its complications can be prevented by taking a modest dose of thiamine, starting even before pregnancy. Since the major effect of thiamine deficiency is dysautonomia, perhaps the exaggeration of asymmetry can be prevented. The new science of epigenetics enables some modification of genetic defects. A mouse model has shown that the combination of a genetic risk factor with short-term gestational hypoxia (oxygen deficit) significantly increases the gene penetrance and severity of vertebral defects. There is no harm in a supplement of thiamine during pregnancy. Whether it would be capable of preventing scoliosis would depend on its disappearance from the medical literature and would be a long-term goal.
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
https://www.scientificanimations.com, CC BY-SA 4.0, via Wikimedia Commons
This article was published originally on August 28, 2017.